Neprilysin breaks down numerous vasoactive peptides. The soluble form of neprilysin, which was recently identified in heart failure, is associated with cardiovascular outcomes. Within a multibiomarker strategy, we directly compared soluble neprilysin and N-terminal pro-B-type natriuretic peptide as risk stratifiers in a real-life cohort of heart failure patients.
MethodsSoluble neprilysin, N-terminal pro-B-type natriuretic peptide, ST2, and high-sensitivity troponin T levels were measured in 797 consecutive ambulatory heart failure patients followed up for 4.7 years. Comprehensive multivariable analyses and soluble neprilysin vs N-terminal pro-B-type natriuretic peptide head-to-head assessments of performance were performed. A primary composite endpoint included cardiovascular death or heart failure hospitalization. A secondary endpoint explored cardiovascular death alone.
ResultsMedian soluble neprilysin and N-terminal pro-B-type natriuretic peptide concentrations were 0.64ng/mL and 1187 ng/L, respectively. Both biomarkers significantly correlated with age (P<.001) and ST2 (P<.001), but only N-terminal pro-B-type natriuretic peptide significantly correlated with estimated glomerular filtration rate (P<.001), body mass index (P<.001), left ventricular ejection fraction (P=.02) and high-sensitivity troponin T (P<.001). In multivariable Cox regression analyses, soluble neprilysin remained independently associated with the composite endpoint (hazard ratio=1.14; 95% confidence interval, 1.02-1.27; P=.03) and cardiovascular death (hazard ratio=1.15; 95% confidence interval, 1.01-1.31; P=.04), but N-terminal pro-B-type natriuretic peptide did not. The head-to-head soluble neprilysin vs N-terminal pro-B-type natriuretic peptide comparison showed good calibration and similar discrimination and reclassification for both neurohormonal biomarkers, but only soluble neprilysin improved overall goodness-of-fit.
ConclusionsWhen added to a multimarker strategy, soluble neprilysin remained an independent prognosticator, while N-terminal pro-B-type natriuretic peptide lost significance as a risk stratifier in ambulatory patients with heart failure. Both biomarkers performed similarly in head-to-head analyses.
Keywords
Heart failure (HF) is a growing public epidemic, with an increasing incidence and prevalence.1 Despite substantial progress in recent decades, mortality remains high for patients with HF. Prognostication may be refined by the use of biomarkers for distinct pathophysiological processes not reflected by established mortality risk factors. In 2008, Braunwald2 classified circulating biomarkers into 7 categories based on their pathophysiological effects in HF and hypothesized that multiple biomarkers in combination would provide a valuable means for risk stratification.3 These pathways include myocardial stretch, myocyte injury, extracellular matrix, inflammation, renal dysfunction, neurohormonal activation, and oxidative stress.
At present, all multimarker approaches include natriuretic peptides as surrogates of the neurohormonal activation pathway.4 However, soluble neprilysin (sNEP) has recently emerged as a potential alternative. Neprilysin (NEP) is a membrane-bound enzyme that cleaves numerous vasoactive peptides, including natriuretic peptides, adrenomedullin, angiotensin-I and -II, bradykinin, and substance P.5,6 This enzyme is fairly ubiquitous and expressed mainly within cell membranes, but a circulating soluble form of NEP was recently reported in HF.7 In an ambulatory cohort of patients with HF, sNEP was found to be an independent predictor of cardiovascular (CV) death and HF hospitalizations.7 By virtue of its central role in neurohormonal regulation, sNEP provides prognostic value on the status of several pathophysiological pathways involved in HF. Therefore, we directly compared sNEP, which is indicative of comprehensive neurohormonal activation, and N-terminal pro-B-type natriuretic peptide (NT-proBNP), a surrogate of natriuretic peptide release and current standard-of-care, in combination with high-sensitivity troponin T (hsTnT) (myocyte injury), ST2 (myocardial fibrosis and inflammation), and estimated glomerular filtration rate (eGFR) (renal dysfunction) for HF prognostication.
METHODSStudy PopulationThe present is a multimarker subanalysis of the previously reported7 total cohort studied. The subanalysis was performed in those patients with availability of all the examined biomarkers (sNEP, NT-proBNP, hsTnT and ST2). From May 2006 to May 2013, ambulatory patients treated at a multidisciplinary HF clinic were consecutively included in the study. Referral inclusion criteria and blood sample collection have been described elsewhere.7,8 All biomarkers were analyzed in the same blood sample stored at −80°C without previous freeze-thaw cycles. All samples were obtained between 09:00 am and 12:00 pm.
All participants provided written informed consent, and the study was approved by the local ethics committee. All study procedures were conducted in accordance with the ethical standards outlined in the 1975 Declaration of Helsinki, as revised in 1983.
Follow-up and OutcomesAll patients were followed-up at regular predefined intervals, with additional visits as required in the case of decompensation. The regular visits schedule included a minimum of quarterly visits with nurses, biannual visits with physicians, and elective visits with geriatricians, psychiatrists, and rehabilitation physicians.9 Patients who did not attend the regular visits were contacted by telephone.
The primary outcome was a composite of CV death or HF hospitalization. Cardiovascular and all-cause deaths were also explored as secondary outcomes. A death was considered CV in origin if it was caused by HF (decompensated HF or treatment-resistant HF in the absence of another cause), sudden death (unexpected death, witnessed or not, of a previously stable patient with no evidence of worsening HF or any other cause of death), acute myocardial infarction (directly related in time to acute myocardial infarction due to mechanical, hemodynamic, or arrhythmic complications), stroke (associated with recently appearing acute neurologic deficit), procedural (post-diagnostic or post-therapeutic procedure death), and other CV causes (eg, rupture of an aneurysm, peripheral ischemia, or aortic dissection). Hospitalizations were identified from the clinic records of patients with HF, hospital wards, and the Catalan electronic medical record. Fatal events were identified from the clinical records of patients with HF, hospital wards, the emergency room, general practitioners, and by contacting the patient's relatives. Data were verified by the databases of the Catalan and Spanish health systems. Events were adjudicated by 2 of the authors (M. Domingo and J. Lupón). Follow-up was closed at March 31, 2014.
Neprilysin AssayHuman NEP was measured using a modified sandwich immunoassay (HUMAN NEP/CD10 ELISA KIT [reference, SK00724-01; lot number, 20111893, Aviscera Biosciences; Santa Clara, United States). Several modifications were made to improve the analytical sensitivity of the method and obtain a lower limit of sample quantification, as reported elsewhere.7 The modified protocol displayed analytical linearity for 0.250-4ng/mL. Samples with concentrations higher than 4ng/mL were diluted to a final concentration between 0.250 ng/mL and 64ng/mL. At a positive control value of 1.4ng/mL, the intra- and inter-assay coefficients of variation were 3.7% and 8.9%, respectively.
N-terminal Pro-B-type Natriuretic Peptide AssayThe NT-proBNP levels were determined using an immuno-electrochemiluminescence method (Elecsys®, Roche Diagnostics; Basel, Switzerland). This assay has < 0.001% cross-reactivity with bioactive B-type natriuretic peptide (BNP), and in the constituent studies in this report, the assay had inter-run coefficients of variation ranging from 0.9% to 5.5%.
High-sensitivity Cardiac Troponin T AssayTroponin levels were measured by electrochemiluminescence immunoassay using an hsTnT assay on the Modular Analytics E 170 (Roche Diagnostics). The hsTnT assay had an analytic range from 3 ng/L to 10000ng/L. At the 99th percentile value of 13 ng/L, the coefficient of variation was 9%. The assays were run with reagents from lot 157123 and were not affected by the analytical issues that have emerged with Roche hsTnT assays.
ST2 AssayST2 was measured from plasma samples using a high-sensitivity sandwich monoclonal immunoassay (Presage® ST2 assay, Critical Diagnostics; San Diego, California, United States). The ST2 assay had a within-run coefficient of < 2.5% and total coefficient of variation of 4%.
Statistical AnalysisCategorical variables are expressed as percentages. Continuous variables are expressed as means ± standard deviation or medians [interquartile range] according to normal or nonnormal distributions. Normal distribution was assessed with normal Q-Q plots. The correlation between sNEP and NT-proBNP concentrations with age, left ventricular ejection fraction, LVEF, eGFR, assessed by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, blood urea and body mass index (BMI) were analyzed using the rho Spearman coefficient due to skewed distribution. Statistical differences (P-value for trend) in NT-proBNP and sNEP concentrations among eGFR subgroups (≥ 60, 30 to < 60, and < 30mL/min/1.73m2) and BMI subgroups (< 20.5, 20.5 to < 25.5, 25.5 to <30, and ≥ 30Kg/m2) were determined using the Spearman test.
Multivariable Cox regression analyses were performed using the backward step method. To fulfill the assumption of linearity of the covariables sNEP, NT-proBNP, hsTnT, and ST2, the logarithmic functions of sNEP, NT-proBNP and hsTnT, and the quadratic term of log (hsTnT) and of ST2 were used in the Cox models. For hazard ratio (HR) calculation in the 3 logarithm-transformed variables, a 1 standard deviation increase was used, and ST2 analyses were performed per every 10ng/mL change. In patients with SNEP levels below the lower range of detection (0.250ng/mL), a concentration of 0.249ng/mL was introduced as a continuous variable. The following variables were incorporated into the model: age, sex, ischemic etiology of HF, LVEF, New York Heart Association functional class, presence of diabetes mellitus, heart rate, systolic blood pressure, hemoglobin, serum sodium, eGFR, NT-proBNP, hsTnT, ST2, beta-blocker therapy, angiotensin-converting enzyme inhibitor or angiotensin receptor blockers therapy, and sNEP. To compare the prognostic values of both sNEP and NT-proBNP, different measurements of performance were used (discrimination, calibration, and reclassification), as described previously.9,10 For calculation of net reclassification improvement, we used risk categories based on tertiles of risk for the composite endpoint (<21%, 21%-39%, and >39%) and CV death (<11%, 11%-23%, and >23%).
Statistical analyses were performed using SPSS 15 (SPSS Inc.; Chicago, Illinois, United States) and the R (version 2.11.1) statistical package (Foundation for Statistical Computing, Vienna, Austria). A 2-sided P < .05 was considered significant.
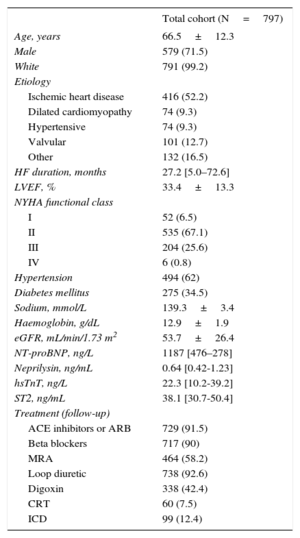
RESULTSCirculating sNEP, NT-proBNP, hsTnT and ST2 were measured in 797 patients with HF who were consecutively enrolled in the study between May 2006 and July 2010. Table 1 provides the baseline characteristics and biomarker metrics of the study cohort. During a mean follow-up period of 4.7±2.4 years, 393 patients died, 216 from CV causes (54.9%), 147 from non-CV causes (37.4%), and 30 from unknown causes (7.6%). Among the known CV causes of death, the cause was refractory HF in 110 (50.9%) patients, sudden death in 47 (21.8%) patients, acute myocardial infarction in 19 (8.8%), stroke in 9 (4.2%), CV procedure in 5 (2.3%) and other CV causes in 26 (12%) patients. During the follow-up period, 193 patients were admitted to the hospital for HF and 300 patients fulfilled the primary endpoint of CV death or HF hospitalization. Five patients were lost to follow-up and adequately censored.
Demographic and Clinical Characteristics at Baseline and Treatments During Follow-up
| Total cohort (N=797) | |
|---|---|
| Age, years | 66.5±12.3 |
| Male | 579 (71.5) |
| White | 791 (99.2) |
| Etiology | |
| Ischemic heart disease | 416 (52.2) |
| Dilated cardiomyopathy | 74 (9.3) |
| Hypertensive | 74 (9.3) |
| Valvular | 101 (12.7) |
| Other | 132 (16.5) |
| HF duration, months | 27.2 [5.0–72.6] |
| LVEF, % | 33.4±13.3 |
| NYHA functional class | |
| I | 52 (6.5) |
| II | 535 (67.1) |
| III | 204 (25.6) |
| IV | 6 (0.8) |
| Hypertension | 494 (62) |
| Diabetes mellitus | 275 (34.5) |
| Sodium, mmol/L | 139.3±3.4 |
| Haemoglobin, g/dL | 12.9±1.9 |
| eGFR, mL/min/1.73 m2 | 53.7±26.4 |
| NT-proBNP, ng/L | 1187 [476–278] |
| Neprilysin, ng/mL | 0.64 [0.42-1.23] |
| hsTnT, ng/L | 22.3 [10.2-39.2] |
| ST2, ng/mL | 38.1 [30.7-50.4] |
| Treatment (follow-up) | |
| ACE inhibitors or ARB | 729 (91.5) |
| Beta blockers | 717 (90) |
| MRA | 464 (58.2) |
| Loop diuretic | 738 (92.6) |
| Digoxin | 338 (42.4) |
| CRT | 60 (7.5) |
| ICD | 99 (12.4) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate (CKD-EPI); HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Data are expressed as no. (%), mean ± standard deviation or median [interquartile range].
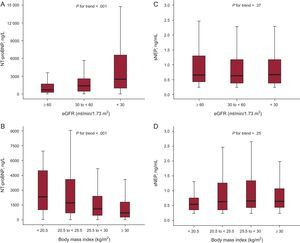
The median soluble sNEP concentration was 0.64ng/mL [0,42-1,23] ng/mL and the median NT-proBNP concentration was 1187 ng/L [476-278] ng/L. sNEP levels were below the analytical measurement range in 101 patients (12.7%). Both biomarkers significantly correlated with age (ρ=0.41, P<.001 and ρ=0.19, P<.001), and NT-proBNP significantly correlated with eGFR rate (ρ=–0.37, P<.001), blood urea (ρ=0.31, P<.001), BMI (ρ=–0.29, P<.001), and weakly with LVEF (ρ=–0.08, P=.02); but sNEP did not (ρ=–0.03, P=.44; ρ=0.002, P=.95; ρ=0.04, P=.23; and ρ=0.008, P=.88, respectively). According to predefined eGFR and BMI strata, the serum concentration of NT-proBNP increased as renal function worsened (Figure 1A) and decreased at higher BMI (Figure 1B), whereas sNEP remained unaffected by these comorbidities (Figures 1C and 1D).
Boxplots for N-terminal pro-B-type natriuretic peptide and soluble neprilysin according to estimated glomerular filtration rate and body mass index subgroups. A: N-terminal pro-B-type natriuretic peptide concentration according to grouped estimated glomerular filtration rate. B: N-terminal pro-B-type natriuretic peptide serum concentration according to grouped body mass index values. C: soluble neprilysin serum concentration according to grouped estimated glomerular filtration rate. D: soluble neprilysin concentration according to grouped body mass index values. The central box represents the values from the lower to the upper quartile and the middle line represents the median. eGFR, estimated glomerular filtration rate; sNEP, soluble neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide. The whiskers extend from the minimum to the maximum value, excluding outside values, which are not displayed.
Moreover, NT-proBNP highly correlated with hsTnT (ρ=0.57, P<.001), and also significantly with ST2 (ρ=0.33, P<.001), whereas sNEP did not correlate with hsTnT (ρ<0.01, P=.83), and correlated modestly with ST2 (ρ=0.15, P<.001).
As previously reported,7 sNEP and NT-proBNP did not correlate in the whole sample (ρ=0.01; P=.68); and in the present multimarker substudy the results remained nonsignificant (ρ=0.04, P=.28).
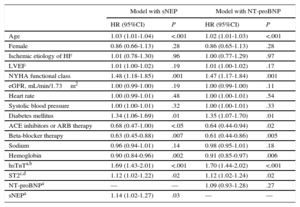
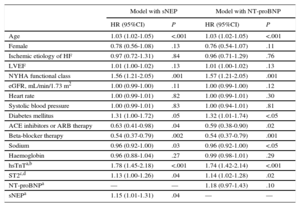
Prognostic Role in OutcomesIn separate Cox regression multivariate analyses that included clinical parameters and the well-recognized HF biomarkers hsTnT and ST2, sNEP remained independently associated with both the composite endpoint (HR=1.14; 95% confidence interval [95%CI], 1.02-1.27; P=.03) (Table 2) and CV death (HR=1.15; 95%CI, 1.01-1.31; P=.04) (Table 3), but NT-proBNP did not, neither for the composite endopint (HR=1.09; 95%CI, 0.93-1.28; P=.27) (Table 2), nor for CV death (HR=1.18; 95%CI, 0.97-1.43; P = .1) (Table 3).
Multivariable Cox Regression Analysis for Risk of the Composite Primary Endpoint (Cardiovascular Death or Heart Failure Hospitalization)
| Model with sNEP | Model with NT-proBNP | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.03 (1.01-1.04) | <.001 | 1.02 (1.01-1.03) | <.001 |
| Female | 0.86 (0.66-1.13) | .28 | 0.86 (0.65-1.13) | .28 |
| Ischemic etiology of HF | 1.01 (0.78-1.30) | .96 | 1.00 (0.77-1.29) | .97 |
| LVEF | 1.01 (1.00-1.02) | .19 | 1.01 (1.00-1.02) | .17 |
| NYHA functional class | 1.48 (1.18-1.85) | .001 | 1.47 (1.17-1.84) | .001 |
| eGFR, mL/min/1.73m2 | 1.00 (0.99-1.00) | .19 | 1.00 (0.99-1.00) | .11 |
| Heart rate | 1.00 (0.99-1.01) | .48 | 1.00 (1.00-1.01) | .54 |
| Systolic blood pressure | 1.00 (1.00-1.01) | .32 | 1.00 (1.00-1.01) | .33 |
| Diabetes mellitus | 1.34 (1.06-1.69) | .01 | 1.35 (1.07-1.70) | .01 |
| ACE inhibitors or ARB therapy | 0.68 (0.47-1.00) | <.05 | 0.64 (0.44-0.94) | .02 |
| Beta-blocker therapy | 0.63 (0.45-0.88) | .007 | 0.61 (0.44-0.86) | .005 |
| Sodium | 0.96 (0.94-1.01) | .14 | 0.98 (0.95-1.01) | .18 |
| Hemoglobin | 0.90 (0.84-0.96) | .002 | 0.91 (0.85-0.97) | .006 |
| hsTnTa,b | 1.69 (1.43-2.01) | <.001 | 1.70 (1.44-2.02) | <.001 |
| ST2c,d | 1.12 (1.02-1.22) | .02 | 1.12 (1.02-1.24) | .02 |
| NT-proBNPa | — | — | 1.09 (0.93-1.28) | .27 |
| sNEPa | 1.14 (1.02-1.27) | .03 | — | — |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; hsTnT, high-sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NEP, neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; sNEP, soluble neprilysin.
High-sensitivity cardiac troponin T as log (hsTnT); N-terminal pro-B-type natriuretic peptide as log (NT-proBNP); neprilysin as log (NEP). For hazard ratio calculation in the 3 logarithm-transformed variables, a 1 standard deviation increase was used.
Multivariable Cox Regression Analysis for Risk of Cardiovascular Death
| Model with sNEP | Model with NT-proBNP | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.03 (1.02-1.05) | <.001 | 1.03 (1.02-1.05) | <.001 |
| Female | 0.78 (0.56-1.08) | .13 | 0.76 (0.54-1.07) | .11 |
| Ischemic etiology of HF | 0.97 (0.72-1.31) | .84 | 0.96 (0.71-1.29) | .76 |
| LVEF | 1.01 (1.00-1.02) | .13 | 1.01 (1.00-1.02) | .13 |
| NYHA functional class | 1.56 (1.21-2.05) | .001 | 1.57 (1.21-2.05) | .001 |
| eGFR, mL/min/1.73 m2 | 1.00 (0.99-1.00) | .11 | 1.00 (0.99-1.00) | .12 |
| Heart rate | 1.00 (0.99-1.01) | .82 | 1.00 (0.99-1.01) | .30 |
| Systolic blood pressure | 1.00 (0.99-1.01) | .83 | 1.00 (0.94-1.01) | .81 |
| Diabetes mellitus | 1.31 (1.00-1.72) | .05 | 1.32 (1.01-1.74) | <.05 |
| ACE inhibitors or ARB therapy | 0.63 (0.41-0.98) | .04 | 0.59 (0.38-0.90) | .02 |
| Beta-blocker therapy | 0.54 (0.37-0.79) | .002 | 0.54 (0.37-0.79) | .001 |
| Sodium | 0.96 (0.92-1.00) | .03 | 0.96 (0.92-1.00) | <.05 |
| Haemoglobin | 0.96 (0.88-1.04) | .27 | 0.99 (0.98-1.01) | .29 |
| hsTnTa,b | 1.78 (1.45-2.18) | <.001 | 1.74 (1.42-2.14) | <.001 |
| ST2c,d | 1.13 (1.00-1.26) | .04 | 1.14 (1.02-1.28) | .02 |
| NT-proBNPa | — | — | 1.18 (0.97-1.43) | .10 |
| sNEPa | 1.15 (1.01-1.31) | .04 | — | — |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; hsTnT, high-sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NEP, neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; sNEP, soluble neprilysin.
High-sensitivity cardiac troponin T as log (hsTnT); N-terminal pro-B-type natriuretic peptide as log (NT-proBNP); neprilysin as log (NEP). For hazard ratio calculation in the 3 logarithm-transformed variables, a 1 standard deviation increase was used.
Neither sNEP (HR=1.02; 95%CI, 0.92-1.14; P=.68) nor NT-proBNP (HR=1.09; 95%CI, 0.95-1.26; P=.23) were independently associated with all-cause death in the multivariate analysis. By contrast, in the analysis focused only on HF endpoints, sNEP remained independently associated with both HF-related death (HR=1.31; 95%CI, 1.11-1.55,; P=.001) and with the composite endpoint of HF-related death or HF hospitalization (HR=1.20; 95%CI, 1.06-1.35; P=.005), whereas NT-proBNP did not (HR=1.18; 95%CI, 0.97-1.43; P=.1 and HR=1.07; 95%CI, 0.89-1.29; P=.48, respectively).
Direct comparison of sNEP vs NT-proBNP in a predictive model containing 11 clinical variables plus hsTnT and ST2 showed no differences in discrimination (all differences in area under the curve, P>.05) (Figure 2) and reclassification (all integrated discrimination improvement and net reclassification improvement, P > .05) for both endpoints added to the reference model (Table 4). The calibration was good, with a nonsignificant Hosmer-Lemeshow test in all models (all P > .05), although the models containing sNEP showed slightly lower Aikaike information criterion and Bayesian information criterion values (better calibration) (data not shown). The addition of sNEP improved overall goodness-of-fit assessed by the likelihood ratio test for both the composite primary endpoint (P=.02) and CV death (P=.03), but NT-proBNP did not (P=.22 and P=.11 respectively). A significant P value in this test means that adding a new variable to the model significantly improves the accuracy of the model of reference.
Area under the curve for the predictive models. Core model (red line): age, sex, ischemic etiology of heart failure, left ventricular ejection fraction, New York Heart Association functional class, presence of diabetes mellitus, hemoglobin, serum sodium, estimated glomerular filtration rate, high-sensitivity troponin T, ST2, beta-blocker therapy, and angiotensin-converting enzyme inhibitor or angiotensin receptot blockers therapy. The model also containing soluble neprilysin is depicted as a green line and the model also N-terminal pro-B-type natriuretic peptide is depicted as a blue line. A: composite endpoint of cardiovascular death and heart failure hospitalization (P=.83 for the comparison between the core model and the model also containing N-terminal pro-B-type natriuretic peptide; P=.24 for the comparison between the core model and the model also containing soluble neprilysin, and P=.22 for the direct comparison between the 2 models containing neurohormonal biomarkers). B: cardiovascular death (P=.87; P=.26, and P=.40 respectively for the same comparisons). AUC, area under the curve sNEP, soluble neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Reclassification for the Composite Endpoint of Cardiovascular Death and Heart Failure Hospitalization and for Cardiovascular Death According to the Addition of Soluble Neprilysin or N-terminal Pro-B-type Natriuretic Peptide-the Core Model
| Composite primary endpoint | Cardiovascular death | |||
|---|---|---|---|---|
| Core model+NT-proBNP | Core model+sNEP | Core model+NT-proBNP | Core model+sNEP | |
| IDI | 0.0 (–0.3 to 0.3); P=.94a | 0.5 (0.0 to 1.0); P=.056a | 0.0 (−0.4 to 0.4); P=.89a | 0.4 (−0.1 to 1.0); P=.12a |
| NRI | 0.3 (–3.1 to 3.6); P=.86a | –2.6 (–6.9 to 1.6); P=.23a | –0.6 (–3.9 to 2.7); P=.73a | –0.7 (–5.0 to 3.7); P=.76a |
| IDI | Reference | 0.5 (–0.1 to 1.1); P=.08b | Reference | 0.4 (–0.2 to 1.0); P=.22b |
| NRI | Reference | –1.5 (–5.7 to 2.7); P=.48b | Reference | –0.7 (–5.8 to 4.3); P=.78b |
IDI, integrated discrimination improvement; NRI, net reclassification improvement; sNEP, soluble neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Core model: age, sex, New York Heart Association functional class, left ventricular ejection fraction, ischemic etiology, diabetes, estimated glomerular filtration rate, hemoglobin, sodium, beta-blocker therapy, angiotensin-converting enzyme inhibitor or angiotensin receptor blockers treatment, ST2, and hsTNT.
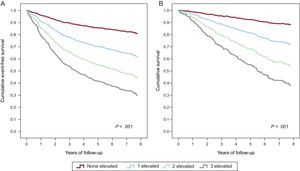
Figure 3 shows the Cox regression event-free survival curve for the primary composite endpoint of CV death or HF hospitalization (Figure 3A) and survival curve for CV death (Figure 3B) according to the serum concentrations below or above the median of sNEP, hsTnT, and ST2.
Cox regression survival curves according to the number of elevated biomarkers (soluble neprilysin, high-sensitivity troponin T and ST2; N=797). A: Event-free survival curve for the primary composite endpoint of cardiovascular death or heart failure hospitalization. B: Survival curve for cardiovascular death. hsTnT, high-sensitivity troponin T; sNEP, soluble neprilysin. A biomarker was considered elevated if equal or above the median values: soluble neprilysin, 0.64 ng/mL; high-sensitivity troponin T, 22.3 ng/L; ST2, 38.1 ng/mL.
Natriuretic peptides have become standard-of-care biomarkers in HF and are currently the only biomarkers that have crossed the research boundary to become routinely used in every day practice. Used as surrogates of myocardial stretch, natriuretic peptides are just one of the multiple counter-regulatory mechanisms activated in HF. In contrast, NEP is an essential enzyme that cleaves a majority of HF-activated neurohormones, including, but not limited to, natriuretic peptides. Therefore, the recent identification of a soluble form of NEP with strong independent prognostic value7 has raised the potential of sNEP as a truly comprehensive neurohormonal biomarker in HF. Here, we performed a head-to-head comparison of both biomarkers within a multimarker strategy that also included ST2 and hsTnT for HF prognostication. Three conclusions emerged from this study. First, both sNEP and NT-proBNP performed similarly in risk stratification in a large cohort with long-term follow-up of real-life patients with HF. Second, sNEP was unaffected by renal dysfunction and BMI. Third, in the context of multimarker analyses, particularly with the incorporation of ST2 and hsTnT, both of which have been shown consistently to be strong prognosticators in chronic HF, only sNEP retained its prognostic value.
Natriuretic peptides were recognized as class IA biomarkers of prognosis in the 2013 American College of Cardiology/American Heart Association HF guidelines.11 A multitude of prospective and retrospective studies have consistently confirmed the usefulness of both BNP and NT-proBNP in predicting risk in HF.12,13 Nevertheless, in the post-PARADIGM era, NT-proBNP may emerge as a stand-alone natriuretic peptide biomarker. Indeed, with the advent of angiotensin receptor and neprilysin inhibitors, particularly after the ground-breaking results of the PARADIGM-HF with LCZ696,14 circulating levels of BNP will almost certainly not be suitable for prognosis, monitoring, and therapeutic guidance. Packer et al15 elegantly demonstrated that treatment with LCZ696 exerts its beneficial effects by inhibiting NEP, which subsequently inhibits BNP degradation, persistently maintaining high BNP levels among treated patients. LCZ696 dissociates the molecular balance between BNP and NT-proBNP such that circulating BNP may no longer reflect the true myocardial stretch, but rather sustained NEP inhibition.15 In contrast, NT-proBNP, not an NEP substrate, exhibited a progressive decline in LCZ696-treated patients as HF improved.
In a recent study,7 our group demonstrated for the first time that high levels of sNEP are found in the circulation of patients with HF and that sNEP concentrations are indicators of adverse outcomes for both CV mortality and morbidity. These data were important for better understanding of sNEP pathobiology in HF and for putting the results obtained in the PARADIGM-HF Trial into context. Nevertheless, the results reported here show that sNEP also provides independent information to other biomarkers commonly used for HF risk stratification and may be an alternative to natriuretic peptides. A current limitation is that the assay used for sNEP determination is not approved for clinical use and requires ad hoc fine-tuning, but it has good intra- and inter-assay coefficients of variation. This assay displays 0% cross-reactivity with the 2 metallopeptidases most similar to this sequence, namely endothelin-converting enzymes 1 and 2, and does not display cross-reactivity with erythrocyte cell-surface antigen (Kell), another protein with strong homology with NEP.16
Renal dysfunction and high BMI are becoming epidemic in HF patients. A recent report from the European Society of Cardiology HF long-term registry indicates that 26.4% of patients hospitalized with HF and 18.2% of chronic HF patients have renal dysfunction and have a median body mass index of 28kg/m2.17 Moreover, data from our group showed that, depending on the equation used to calculate estimated glomerular filtration rate, the prevalence of renal failure (defined as eGFR < 60 mL/min/1.73 m2) may be as high as 64% in ambulatory patients with chronic HF.18 The use of a biomarker significantly affected by these comorbidities may hamper widespread clinical use for prognostication. Given that plasma NT-proBNP is excreted by the kidney, we found, as expected, that decreased renal function is independently associated with higher plasma NT-proBNP concentrations. Previous studies have suggested that plasma NT-proBNP concentrations increase in patients with kidney dysfunction due to impaired clearance19; however, other studies have suggested that this finding may be explained by increased cardiac secretion due to coexistent CV disease.20 In this study, sNEP was unaffected by renal function, but many unsettled issues remain and the exact excretion mechanism of sNEP is currently uncertain. In regards to BMI, an important clinical factor influencing outcomes in HF patients,21,22 sNEP concentration remained unaffected across BMI strata, whereas NT-proBNP showed a well-recognized reduction at higher BMI.23 Taken together, our data indicate that sNEP is a novel independent prognostic biomarker that does not require adjustments for most common HF comorbidities.
Interest is increasing in multimarker strategies to examine panels of biomarkers that assess different pathophysiological pathways.24 Several recently reported scores for risk prediction assessment have shown that multiple biomarker scoring is superior to a conventional risk score including clinical parameters and NT-proBNP.25,3 Additional predictive information from different biological pathways reflects the multisystemic character of HF. In this study, we examined the value of ST2, which is indicative of fibrosis,26 and hsTnT, which is reflective of myocyte injury27 in combination with sNEP or NT-proBNP, which are both indicative of neurohormonal activation, using a comprehensive clinical model including 11 prognostically meaningful variables (age, sex, ischemic etiology of HF, LVEF, New York Heart Association, NYHA functional class, diabetes mellitus, hemoglobin, sodium, eGFR, beta blockers, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy). Remarkably, sNEP retained its predictive value in combination with ST2 + hsTnT for CV- and HF-related outcomes, whereas NT-proBNP was no longer relevant. A number of biomarker panels may perform equivalently; choosing which one to employ in clinical practice will depend on factors such as cost, ease of assay, and potential therapeutic implications. Our present multimarker substudy, beyond the description of sNEP as a valuable biomarker in HF recently described,7 suggests that the triad ST2, hsTnT, and sNEP may eventually become a panel of choice once the sNEP assay is refined.
Neither sNEP nor NT-proBNP were independently associated with all-cause death in the multivariate analysis. It should be taken into account that non-CV death was not negligible (37.4% of patients) and this might have influenced the results. We chose the same endpoints as the PARADIGM-HF Trial, which are the most accepted in recent HF trials. Remarkably, sNEP also remained independently associated with HF-related death and HF hospitalization whereas NT-proBNP did not.
LimitationsThe experimental assay for sNEP measurement described here has long incubation times, making it ill-suited for daily clinical use. We have no data on the stability of sNEP while frozen so we cannot rule out the possibility that the sNEP concentrations found would have been different in fresh samples. Samples were obtained during routine visits and no data on the clinical stability of patients (ie, possible decompensation during the 3 previous months) were collected. However, the sample is representative of ambulatory chronic HF patients in real life. Although the study population was a real-life HF population with different HF etiologies, it was treated at a specific multidisciplinary HF unit in a tertiary care hospital; most patients were referred from the cardiology department and, thus, were relatively young men with HF of ischemic etiology and reduced LVEF. As such, these results cannot necessarily be extrapolated to a more global HF population. In the near future, with the likely widespread use of NEP inhibitors in patients with HF and reduced LVEF, the prognostic value and use of sNEP and other circulating biomarkers may change.
Prospective studies are needed to assess tailored strategies for pharmacological NEP inhibition based on measurements of sNEP levels in patients with HF. The appropriate use of biomarkers supporting management of patients with HF should help to reduce the costs of a very costly disease in developed countries.28
CONCLUSIONSWhen added to a multimarker strategy that also incorporates ST2 and hsTnT, sNEP remained an independent prognosticator while NT-proBNP lost significance as risk stratifier in ambulatory patients with HF. In head-to-head analyses, sNEP performed similarly to NT-proBNP, but it was less influenced by comorbidities (renal function and BMI).
CONFLICTS OF INTERESTA. Bayes-Genis and J. Lupón have received lecture honoraria from Roche Diagnostics and A. Bayes-Genis from Critical Diagnostics. A. Bayes-Genis and J. Lupón report a relationship with Critical Diagnostics.
We thank Beatriz González, Roser Cabanes, Margarita Rodríguez, Nuria Benito and Alba Ros for data collection and invaluable work in the HF Clinic.
We also wish to acknowledge Redes Temáticas de Investigación Cooperativa en Salud, Red de Investigación Cardiovascular (RD12/0042/0047; postdoctoral fellowship), Red de Terapia Celular (RD12/0019/0029), and Ministerio de Economía y Competitividad (Juan de la Cierva, JCI-2012-14025).