The optimal treatment of patients with multivessel coronary artery disease and ST-segment elevation acute myocardial infarction (STEMI) who undergo primary percutaneous coronary intervention (PCI) is controversial. The aim of this study was to access the prognostic impact of multivessel PCI vs culprit vessel-only PCI in real-world patients with STEMI and multivessel disease.
MethodsThis was a retrospective cohort study of 1499 patients with STEMI diagnosis who underwent primary PCI between January 2008 and December 2015. About 40.8% (n=611) patients had multivessel disease. We performed a propensity score matched analysis to obtain 2 groups of 215 patients paired according to whether or not they had undergone multivessel PCI or culprit vessel-only PCI.
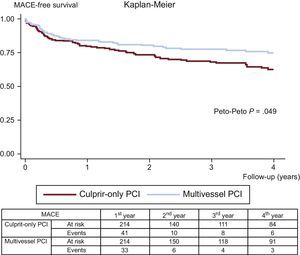
ResultsDuring follow-up (median, 2.36 years), after propensity score matching, patients who underwent multivessel PCI had lower rates of mortality (5.1% vs 11.6%; Peto-Peto P=.014), unplanned repeat revascularization (7.0% vs 12.6%; Peto-Peto P=.043) and major acute cardiovascular events (22.0% vs 30.8%; Peto-Peto P=.049). These patients also showed a trend to a lower incidence of myocardial infarction (4.2% vs 6.1%; Peto-Peto P=.360).
ConclusionsIn real-world patients presenting with STEMI and multivessel coronary artery disease, a multivessel PCI strategy was associated with lower rates of mortality, unplanned repeat revascularization, and major acute cardiovascular events.
Keywords
Multivessel coronary artery disease (MVD) is a frequent angiographic finding in ST-segment elevation acute myocardial infarction (STEMI), occurring in more than 40% of patients undergoing primary percutaneous coronary intervention (PCI).1,2 Compared with patients with single-vessel disease, those with MVD have worse in-hospital and long-term prognosis, including repeat admissions for myocardial infarction and revascularization procedures.2–4 To overcome this scenario, the concept of preventive nonculprit lesion PCI has emerged as an alternative to the traditional strategy of infarct-related artery only revascularization. However, undertaking PCI in nonculprit lesions can have potential complications. Current European Society of Cardiology guidelines recommend that primary PCI should be limited to the culprit vessel (with the exception of cardiogenic shock and persistent ischemia) and that staged revascularization of nonculprit lesions should be considered if there are symptoms or ischemia within days to weeks after primary PCI.5,6 Recent published trials, such as PRAMI,7 CvLPRIT8 and DANAMI-3 PRIMULTI,9 have questioned the need, timing, and criteria to perform multivessel revascularization in patients with STEMI, showing better outcomes with complete immediate or staged revascularization.
To define the impact of multivessel PCI vs culprit-only PCI in real-world patients, we analyzed our 8-year retrospective registry with 1499 STEMI patients undergoing primary PCI.
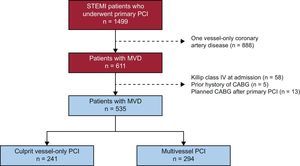
METHODSStudy PopulationThis was a retrospective single-center observational cohort study that enrolled consecutive patients with a diagnosis of STEMI who underwent primary PCI at the University Clinical Hospital of Santiago de Compostela, Spain, between January 2008 and December 2015 (n=1499). About 40.8% (n=611) patients had MVD. To analyze the impact of multivessel vs culprit vessel-only PCI, patients with MVD and the following characteristics were excluded: a) Killip class IV at admission (n=58), b) a prior history of coronary artery bypass grafting (n=5), and c) planned coronary artery bypass grafting after primary PCI (n=13), as illustrated in Figure 1. The study population consisted of 535 patients with MVD, of which 55.0% (n=294) underwent multivessel PCI and 45.0% (n=241) underwent culprit vessel-only PCI. The decision to perform nonculprit coronary artery percutaneous revascularization and its timing were left to the discretion of the interventional cardiologist and clinical cardiologist or to the Heart Team, when appropriate. The factors influencing this decision were recorded.
Primary PCI was undertaken according to the European Society of Cardiology guidelines10,11 and the operators’ routine practice and could include aspiration thrombectomy, heparin, or glycoprotein IIb/IIIa inhibitor administration. Antiplatelet therapy included aspirin and a P2Y12 inhibitor (clopidogrel in the first few years and ticagrelor and prasugrel more recently).
Demographic, clinical, echocardiographic, coronary angiographic, and laboratory data at admission were collected and recorded in a computerized database, in accordance with the protocol in our department for patients with STEMI undergoing primary PCI. Glomerular filtration rate was calculated at admission using the Cockgrauft-Gault formula.
DefinitionsDiagnosis of STEMI was made according to current guidelines.11,12 Ischemia time was defined as the time between symptom onset and reperfusion (guide wire passage in the culprit artery during primary PCI). The MVD was defined as at least 1 lesion in a noninfarct-related artery deemed angiographically significant (more than 50% luminal narrowing diameter). Culprit vessel-only PCI was defined as revascularization of only the infarct-related artery, and multivessel PCI as revascularization of at least 1 more lesion of a different vessel during the index procedure or scheduled for the following 30 days. Major acute cardiovascular events (MACE) during follow-up were composed of all-cause mortality, myocardial infarction, heart failure requiring hospitalization, and unplanned repeat revascularization.
Study Aim and Follow-upThe primary aim of this study was to compare the clinical outcomes during follow-up (all-cause mortality and MACE) of multivessel PCI vs culprit vessel-only PCI in patients with STEMI undergoing primary PCI and MVD, after adjustment with propensity score matching.
Follow-up was performed through consultation of the electronic registries available in the autonomous community of Galicia (IANUS program); all medical evaluations and hospital registries were reviewed. Median follow-up was 2.36 years (interquartile range, 0.68-4.67 years).
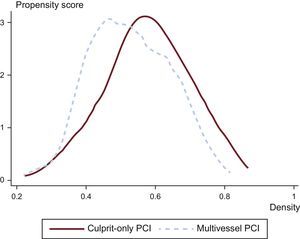
Statistical AnalysisUnivariate analysis was performed of categorical variables using the chi-square test, with results expressed as percentages and of continuous variables using the Student t test, with results expressed as means±standard deviation. As this was a nonrandomized observational study, propensity score matching was conducted to match the study populations (multivessel PCI vs culprit vessel-only PCI patients) and to reduce the bias due to confounding variables that could influence treatment decisions and clinical outcomes.13 Matching was performed using the following variables: age, sex, body mass index, hypertension, diabetes, dyslipidemia, smoking, ischemic cardiomyopathy, ischemia time, STEMI localization (anterior vs other), infarct-related artery, use of drug-eluting stents, use of glycoprotein IIb/IIIa inhibitors, number of diseased vessels (2-vessel vs 3-vessel coronary artery disease), glomerular filtration rate, creatinine, troponin I peak, hemoglobin, glucose, heart rate, systolic blood pressure, Killip class, left ventricular ejection fraction, GRACE score, CRUSADE score, and the year of the patient's entry. To estimate the propensity score of each patient, matching was based on the nearest neighbor technique without replacement by means of a probit model using all the variables mentioned above. This method is based on the identification of patients inside the common support area of the 2 groups. The degree of overlap in the area of common support for the propensity score matching was very high, as shown in Figure 2, which allowed the inclusion of 215 patients in each group. There were no statistically significant differences in baseline characteristics between multivessel and culprit vessel-only PCI patients. The goodness-of-fit of the propensity matching score was adequate (Hosmer-Lemeshow test chi-square=476.44) and the variables used showed no problems of multicollinearity (mean variance inflation factor=2.01).
Kaplan-Meier analysis with a modified log-rank test was used to illustrate 4-year cumulative all-cause mortality for patients depending on the revascularization strategy (culprit vessel-only vs multivessel PCI). Kaplan-Meier mortality curves showed some superimposition during the first few months of follow-up and therefore the Peto-Peto-Prentice test, a statistical test assigning more weight to earlier events, was used to confirm the results. Kaplan-Meier analysis with Peto-Peto-Prentice tests was also performed to evaluate differences in MACE during follow-up. To avoid violation of proportionality assumptions both in the analysis of all-cause mortality and MACE, previous studies have proposed a restricted mean survival analysis.14,15 Consequently, we used the model proposed by Royston and Parmar,16 which shows the number of days gained until each event during follow-up and its statistical significance.
Since the nonfatal components of MACE (myocardial infarction, heart failure, and repeat revascularization) and the endpoint of stent thrombosis may compete with all-cause mortality,17,18 we used a competing risk model19 that shows the cumulative incidence function curves for such endpoints and their sub-hazard ratio differences.
All analyses were 2-tailed and differences were considered significant if the P value was<.05. The statistical analysis was carried out using SPSS version 22.0.
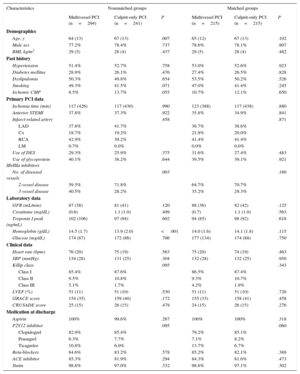
RESULTSBaseline CharacteristicsThe initial cohort was composed of 535 patients with MVD; mean age was 65.8±13.0 years, 77.8% were male, 27.7% had diabetes, and 37.6% presented with anterior STEMI. We compared baseline characteristics between patients who underwent multivessel PCI (55.0%; n=294) and culprit vessel-only PCI (45.0%; n=241). Multivessel PCI was mostly performed as a staged procedure (85.4%; n=251); multivessel revascularization during the primary PCI procedure was accomplished in 43 patients (14.6%). Complete revascularization was achieved in 67.3% patients (n=198) who underwent multivessel PCI. The decision not to perform PCI of nonculprit coronary arteries was influenced by many factors (Table 1). Multivessel PCI patients were younger, had higher levels of hemoglobin at admission, and more often had 3-vessel disease on coronary angiography (Table 2). Patients who underwent multivessel PCI were less likely to have previous history of ischemic cardiomyopathy (8.5% vs 13.7%; P=.055) and were more likely to have higher glomerular filtration rate at admission (87 vs 81mL/min; P=.120), although these differences were not statistically significant. There were no significant differences regarding sex, cardiovascular risk factors, ischemia time, infarct-related artery, use of drug-eluting stents during primary PCI, troponin peak, hemodynamic variables, Killip class, left ventricular ejection fraction, and risk scores (GRACE and CRUSADE), as depicted in Table 2. Pharmacological treatment at hospital discharge was similar between groups.
Factors Influencing the Decision Not to Perform Multivessel-percutaneous Coronary Intervention
| Factors | n (%) |
|---|---|
| Residual moderate lesion (≤ 70% stenosis) | 97 (40.2%) |
| Complex residual lesion/anatomic difficulties | 65 (27.0%) |
| Chronic total occlusion | 44 (18.3%) |
| Negative ischemia stress test | 35 (14.5%) |
Baseline Characteristics of the Study Groups
| Characteristics | Nonmatched groups | Matched groups | ||||
|---|---|---|---|---|---|---|
| Multivessel PCI (n=294) | Culprit-only PCI (n=241) | P | Multivessel PCI (n=215) | Culprit-only PCI (n=215) | P | |
| Demographics | ||||||
| Age, y | 64 (13) | 67 (13) | .007 | 65 (12) | 67 (13) | .102 |
| Male sex | 77.2% | 78.4% | .737 | 78.6% | 78.1% | .907 |
| BMI, kg/m2 | 29 (5) | 28 (4) | .437 | 29 (5) | 28 (4) | .482 |
| Past history | ||||||
| Hypertension | 51.4% | 52.7% | .758 | 53.0% | 52.6% | .923 |
| Diabetes mellitus | 28.9% | 26.1% | .476 | 27.4% | 26.5% | .828 |
| Dyslipidemia | 50.3% | 49.8% | .654 | 53.5% | 50.2% | .526 |
| Smoking | 49.3% | 41.5% | .071 | 47.0% | 41.4% | .245 |
| Ischemic CMP | 8.5% | 13.7% | .055 | 10.7% | 12.1% | .650 |
| Primary PCI data | ||||||
| Ischemia time (min) | 117 (426) | 117 (430) | .990 | 123 (388) | 117 (438) | .880 |
| Anterior STEMI | 37.8% | 37.3% | .922 | 35.8% | 34.9% | .841 |
| Infarct-related artery | .458 | .871 | ||||
| LAD | 37.8% | 41.7% | 36.7% | 38.6% | ||
| Cx | 18.7% | 19.2% | 21.9% | 20.0% | ||
| RCA | 42.9% | 39.2% | 41.4% | 41.4% | ||
| LM | 0.7% | 0.0% | 0.0% | 0.0% | ||
| Use of DES | 29.3% | 25.9% | .375 | 31.6% | 27.4% | .483 |
| Use of glycoprotein IIb/IIIa inhibitors | 40.1% | 38.2% | .644 | 39.5% | 39.1% | .921 |
| No. of diseased vessels | .003 | .180 | ||||
| 2-vessel disease | 59.5% | 71.8% | 64.7% | 70.7% | ||
| 3-vessel disease | 40.5% | 28.2% | 35.2% | 29.3% | ||
| Laboratory data | ||||||
| GFR (mL/min) | 87 (38) | 81 (41) | .120 | 88 (36) | 82 (42) | .125 |
| Creatinine (mg/dL) | (0.6) | 1.1 (1.0) | .499 | (0.7) | 1.1 (1.0) | .563 |
| Troponin I peak (ng/mL) | 102 (106) | 97 (94) | .602 | 94 (85) | 98 (92) | .618 |
| Hemoglobin (g/dL) | 14.5 (1.7) | 13.9 (2.0) | <.001 | 14.0 (1.6) | 14.1 (1.8) | .115 |
| Glucose (mg/dL) | 174 (87) | 172 (86) | .706 | 177 (134) | 174 (88) | .750 |
| Clinical data | ||||||
| Heart rate (bpm) | 76 (20) | 75 (19) | .563 | 75 (20) | 74 (19) | .463 |
| SBP (mmHg) | 134 (28) | 131 (25) | .304 | 132 (28) | 132 (25) | .950 |
| Killip class | .095 | .343 | ||||
| Class I | 85.4% | 87.6% | 86.5% | 87.4% | ||
| Class II | 9.5% | 10.8% | 9.3% | 10.7% | ||
| Class III | 5.1% | 1.7% | 4.2% | 1.9% | ||
| LVEF (%) | 51 (11) | 51 (10) | .530 | 51 (11) | 51 (10) | .726 |
| GRACE score | 154 (35) | 159 (40) | .172 | 155 (33) | 158 (41) | .458 |
| CRUSADE score | 25 (15) | 26 (15) | .478 | 24 (15) | 26 (15) | .276 |
| Medication at discharge | ||||||
| Aspirin | 100% | 99.6% | .267 | 100% | 100% | .318 |
| P2Y12 inhibitor | .095 | .060 | ||||
| Clopidogrel | 82.9% | 85.4% | 79.2% | 85.1% | ||
| Prasugrel | 6.3% | 7.7% | 7.1% | 8.2% | ||
| Ticagrelor | 10.8% | 6.0% | 13.7% | 6.7% | ||
| Beta-blockers | 84.6% | 83.2% | .578 | 85.2% | 82.1% | .389 |
| ACE inhibitor | 85.3% | 81.9% | .294 | 84.3% | 81.6% | .473 |
| Statin | 98.6% | 97.0% | .332 | 98.6% | 97.1% | .302 |
ACE inhibitor, angiotensin-converting enzyme inhibitor; BMI, body mass index; bpm, beats per minute; CMP, cardiomyopathy; Cx, circumflex artery; DES, drug-eluting stent; GFR, glomerular filtration rate; LAD, left anterior descending artery; LM, left main coronary artery; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA: right coronary artery; SBP: systolic blood pressure; STEMI: ST-elevation acute myocardial infarction.
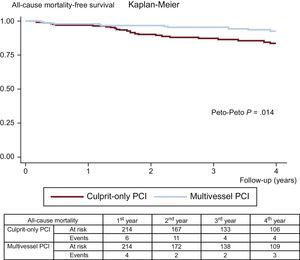
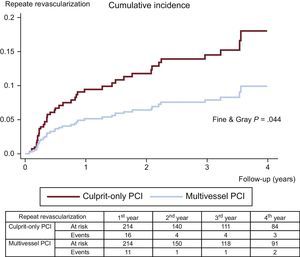
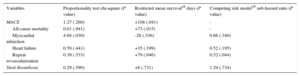
The clinical adverse events during follow-up are displayed in Table 3. After propensity score matching, all-cause mortality (11.6% vs 5.1%; Peto-Peto P=.014; Figure 3), unplanned repeat revascularization (12.6% vs 7.0%; Peto-Peto P=.043; Figure 4) and MACE (30.8% vs 22.0%; Peto-Peto P=.049; Figure 5) remained significantly higher in patients who underwent culprit vessel-only PCI. These patients also had a trend toward a higher incidence of myocardial infarction (6.1% vs 4.2%; Peto-Peto P=.360) and heart failure (5.1% vs 2.8%; Peto-Peto P=.187). Tests to evaluate the robustness of the data confirmed the former results (Table 4). The restricted mean survival analysis demonstrated a significant gain in days until the occurrence of MACE, all-cause mortality, and repeat revascularization. The competing model demonstrated that repeat revascularization was almost halved (subhazard ratio=0.52, P=.044; Figure 4).
Adverse Events During Follow-up
| Variables | Nonmatched groups | Matched groups | ||||
|---|---|---|---|---|---|---|
| Multivessel PCI (n=294) Median time to event (IQR) | Culprit-only PCI (n=241) Median time to event (IQR) | Peto-Peto P | Multivessel PCI (n=214) Median time to event (IQR) | Culprit-only PCI (n=214) Median time to event (IQR) | Peto-Peto P | |
| MACE | 29.9% | 34.4% | .016 | 22.0% | 30.8% | .049 |
| 153 (351) | 201 (523) | 129 (362) | 166 (525) | |||
| All-cause mortality | 7.5% | 13.3% | .014 | 5.1% | 11.6% | .014 |
| 292 (697) | 590 (479) | 439 (1070) | 590 (398) | |||
| Myocardial infarction | 5.5% | 7.5% | .215 | 4.2% | 6.1% | .360 |
| 181 (259) | 309 (748) | 84 (160) | 523 (745) | |||
| Heart failure | 4.4% | 5.5% | .198 | 2.8% | 5.1% | .187 |
| 118 (255) | 91 (178) | 130 (583) | 107 (279) | |||
| Repeat revascularization | 9.6% | 14.2% | .014 | 7.0% | 12.6% | .043 |
| 181 (255) | 313 (624) | 179 (433) | 309 (685) | |||
| Stent thrombosis | 2.4% | 2.1% | .695 | 1.9% | 1.4 | .731 |
| 39 (359) | 74 (70) | 22 (197) | 74 (70) | |||
IQR, interquartile range; MACE, major acute cardiovascular events; PCI, percutaneous coronary intervention.
Robustness Check Using a Restricted Mean Survival Analysis and a Competing Risk Model
| Variables | Proportionality test chi-square (P value) | Restricted mean survival16 days (P value) | Competing risk model19 sub-hazard ratio (P value) |
|---|---|---|---|
| MACE | 1.27 (.260) | +108 (.041) | - |
| All-cause mortality | 0.01 (.941) | +73 (.015) | - |
| Myocardial infarction | 4.68 (.030) | -28 (.336) | 0.66 (.340) |
| Heart failure | 0.59 (.441) | +35 (.199) | 0.52 (.195) |
| Repeat revascularization | 0.39 (.533) | +79 (.046) | 0.52 (.044) |
| Stent thrombosis | 0.29 (.590) | +6 (.731) | 1.29 (.734) |
MACE, major acute cardiovascular events.
The present study supports a beneficial effect of multivessel PCI in patients with STEMI and MVD compared with the culprit vessel-only PCI strategy, after adjustment of baseline characteristics with propensity score matching. In this real-world all-comers study, we report a significant reduction in all-cause mortality, unplanned repeat revascularization, and MACE during follow-up with data supported by a robust statistical analysis.
In this study, the decision regarding the performance of multivessel PCI was left to the discretion of the clinical and interventional cardiologists or to the Heart Team, when appropriate. This reflects real-world practice, where the decision of how, when, and which coronary arteries should be revascularized or not, is individualized attending to the anatomical characteristics of the remaining lesions, symptoms, results of ischemia stress tests, myocardial territory at risk, left ventricular systolic function, risk of complications, center/operator experience, and patient age, comorbidities, and preference. The integration of all these factors, a complex process that can hardly be evaluated in a single randomized controlled trial, may favor one strategy over another (medical treatment vs partial or complete revascularization).
The increased morbidity and mortality risk in patients with STEMI and MVD compared with those with 1-vessel disease can be explained by several mechanisms including multiple plaque instability, impaired myocardial perfusion and contractility, arrhythmia, and death.1,20 The potential advantages of multivessel PCI in this context include the prevention of recurrent ischemia/myocardial infarction and its associated complications, reduction of jeopardized myocardial territory, and improvement of myocardial function due to better flow to periinfarct areas.21 However, multivessel PCI might also have disadvantages: longer procedure time, increased use of contrast dye, higher radiation dose exposure and an increase in stent-related complications (stent thrombosis and restenosis) due to additional stent implantation. New antiplatelet agents,22,23 radial access,24,25 and new generation drug-eluting stents26,27 may contribute to a safer procedure for expert operators. In our study, patients who underwent multivessel PCI had a similar rate of stent thrombosis compared with culprit vessel-only PCI patients. The apparently high rate of stent thrombosis (> 2.0% in each group before matching) might be related to the long-term follow-up (including cases of very late stent thrombosis), the use of older stents in the first few years, and the inclusion of elderly patients with many comorbidities.
In the present study, multivessel PCI was mainly performed as a staged procedure and seldom at the time of primary PCI, in keeping with current recommendations.5,11 Intervention of a nonculprit lesion in a single procedure after primary PCI may result in unnecessary hemodynamic compromise at a time when the patient has significant regional myocardial compromise due to infarction and stunning; besides, nonculprit lesions may be overestimated (leading to unnecessary PCI)28 and their physiologic significance is often difficult to evaluate. Staged-PCI allows evaluation of the physiological significance of nonculprit lesions (either noninvasively or via fractional flow reserve), provides time for discussion of revascularization strategies, and is probably safer.29
Recently, 3 randomized trials have been published on the management of MVD after primary PCI. The PRAMI trial7 indicated that preventive PCI of noninfarct coronary arteries with angiographically significant lesions at the index procedure significantly reduced the risk of nonfatal myocardial infarction and refractory angina compared with PCI limited to the infarct artery. In addition, CvLPRIT8 demonstrated that complete revascularization during the index admission resulted in a significantly lower MACE rate at 12 months (when the components were evaluated separately, there was only a trend to lower mortality, reinfarction, heart failure, and repeat revascularization) than when only the infarct-related artery was treated. Finally, the DANAMI-3 PRIMULTI9 showed that complete revascularization guided by fractional flow reserve measurements significantly reduced the risk of adverse events compared with no further invasive intervention after primary PCI; this effect was mainly driven by significantly fewer repeat revascularizations. Additionally, complete revascularization appears to be safe, since a pooled analysis of this study did not show an increase in cerebrovascular accidents, bleeding or contrast-induced nephropathy.8,30 As a consequence, recent published meta-analyses of randomized clinical trials and observational studies show better outcomes in favor of multivessel revascularization in patients with STEMI31–34; however, there is only firm evidence for reduction of MACE (largely driven by reduction in repeat revascularization), without solid evidence for the reduction in death or myocardial infarction. In our study, we also found a reduction in MACE and repeat revascularization; notwithstanding, mortality was also lower in patients who underwent multivessel PCI. Real-world patients are older, have more comorbidities and worse prognosis, which might have contributed, together with the long follow-up, to our findings on mortality. Currently, there is an ongoing large randomized clinical trial (COMPLETE) powered for the hard outcomes of death and myocardial infarction that is enrolling patients with STEMI to culprit-only revascularization or staged complete revascularization preferably performed during the index hospitalization; this trial encourages second generation drug-eluting stents and dual antiplatelet therapy with ticagrelor, reflecting contemporary practice.
LimitationsThere are several limitations to be considered in the interpretation of our study. First, this was a retrospective observational and nonrandomized study conducted at a single hospital and, as such, has the inherent limitations and bias related to retrospective single-center studies. Although propensity score matching between groups increases the strength of statistical analyses, it is impossible to correct for unmeasured confounding factors and all the selection biases regarding treatment decision, which precludes definite conclusions. Second, the factors that led to the performance of multivessel PCI in each case could not be ascertained and, consequently, it is not possible to define a standard approach to patients with MVD and STEMI. Third, we did not collect data on functional assessment of coronary stenosis in patients who underwent multivessel PCI; it is likely that, in most patients, assessment of nonculprit lesions significance was made only on angiography.
CONCLUSIONSOur study supports the recent findings of randomized clinical trials and meta-analyses in a real-world population, showing that multivessel PCI of nonculprit lesions in patients with a diagnosis of STEMI may reduce adverse clinical events during follow-up, including MACE, unplanned repeat revascularization and all-cause mortality.
CONFLICTS OF INTERESTNone declared.
- -
No consensus exists regarding the management of multivessel disease detected at the time of primary PCI, unless the patient is in cardiogenic shock or has persistent ischemia.
- -
Current guidelines recommend staged revascularization of nonculprit lesions only if the patient has symptoms or ischemia is detected after primary PCI.
- -
Recent randomized clinical trials have shown better clinical outcomes associated with complete immediate or staged revascularization than with culprit lesion-only PCI.
- -
This study supports the recent findings of clinical trials, showing that multivessel PCI of nonculprit lesions in real-world patients with a diagnosis of STEMI may reduce adverse clinical outcomes during follow-up.