Computed tomography does not accurately determine which coronary lesions lead to myocardial ischemia and consequently further tests are required to evaluate ischemia induction. The aim of this study was to compare diagnostic accuracy between dual-energy computed tomography and magnetic resonance imaging in the assessment of myocardial perfusion and viability in patients suspected of coronary artery disease.
MethodsA prospective study was performed in 56 consecutive patients (39 men [69.6%]; mean age [standard deviation], 63 [10]; range, 23-81). Computed tomography was performed with the following protocol: 1, adenosine stress perfusion; 2, coronary angiography; and 3, delayed enhancement. Magnetic resonance imaging for the evaluation of stress perfusion and delayed enhancement was performed within 30 days. Two observers in consensus analyzed the perfusion and delayed enhancement images.
ResultsWe studied 952 myocardial segments and 168 vascular territories. In a per-segment analysis, the sensitivity, specificity, and positive and negative predictive values of computed tomography compared with magnetic resonance were 76%, 99%, 89%, and 98% for perfusion defects, and 64%, 99%, 82%, and 99% for delayed enhancement, respectively. In a per-vascular territory analysis, the same measures were 78%, 97%, 86%, and 95% for perfusion defects, and 72%, 99%, 93%, and 97% for delayed enhancement, respectively. The mean radiation dose was 8.2 (2) mSv.
ConclusionsDual-source computed tomography may allow accurate and concomitant evaluation of perfusion defects and myocardial viability and analysis of coronary anatomy.
Keywords
Coronary computed tomography (CT) angiography is a useful technique for evaluating the coronary arteries of patients without known coronary artery disease. Its high negative predictive value allows coronary artery disease to be safely ruled out in patients with normal test results.1 However, it is difficult to predict which stenoses, when present, will result in a deterioration in myocardial flow,2 particularly when there are calcifications of significant or intermediate severity. Thus, stenosis quantification is not a certain indicator of myocardial ischemia and the role of imaging in the diagnosis of myocardial ischemia is incomplete without verification of the functional limitation caused by coronary stenoses.3
The hemodynamic status of coronary artery disease can be evaluated with myocardial perfusion imaging (MPI) during stress induced by exercise or drugs.4 Techniques used to detect myocardial perfusion defects in routine clinical practice are single-photon emission computed tomography (SPECT) and magnetic resonance (MR) imaging. Of these imaging modalities, the latter has the added value of obtaining delayed enhancement images that help diagnose myocardial necrosis.5–7
Recent studies have shown that myocardial ischemia can be assessed by MPI with CT and that the results agree with those of SPECT and conventional coronary angiography.8–10 Dual-source CT (DSCT), which has 2 X-ray tubes with corresponding detector arrays, has important advantages, namely the ability to perform high pitch and low radiation dose11 and dual-energy CT (DECT) acquisitions in which each X-ray tube operates at a different energy. This technique can generate color maps that allow the evaluation of the myocardial perfusion status via the analysis of the iodine volume in the myocardium.
Recent studies of MPI with DECT have demonstrated high accuracy in the detection of myocardial ischemia.12 Moreover, the combination of MPI with DECT increases the diagnostic value of coronary CT angiography for the detection of significant coronary stenoses.13 Furthermore, high-pitch acquisition with a low radiation dose has shown good accuracy in the assessment of myocardial viability in comparison with MR imaging, despite an increase in image “noise”.14 The ability of this imaging modality to simultaneously obtain data on coronary anatomy, functional effects of lesions, and myocardial necrosis-viability in a single test could be the desired “one-stop shop” for complementary tests for coronary artery disease.
The aim of this study was to compare the diagnostic accuracy of DSCT with that of MR imaging in the assessment of myocardial perfusion and viability in patients with clinical suspicion of ischemic heart disease.
METHODSStudy PopulationThis was a prospective study that included those patients who attended a cardiology clinic with clinical suspicion of ischemia and with positive stress test results. All patients were suggested to undergo DSCT and cardiac MR imaging with an interval of <30 days between tests. We excluded patients with asthma, allergy to iodine or gadolinium contrast agent, irregular heart rate, pacemaker, renal failure with a glomerular filtration rate<60 mL/min, hypertrophic cardiomyopathy, dilated cardiomyopathy with a left ventricular ejection fraction (LVEF)>30%, or functional class III or IV heart failure.
The study protocol was approved by the institutional ethics committee and all patients gave written informed consent before inclusion.
Imaging StudiesCardiac Computed Tomography ProtocolPatients were instructed to avoid coffee, tea, and oral beta-blockers in the 24h before the scan. Two venous access sites (18-G for contrast administration, 20-G for adenosine infusion) were placed in the right arm. Heart rate and blood pressure were monitored.
All scans were performed on a 128-DSCT system (Flash Definition®; Siemens, Forcheim, Germany) with the following protocol:
Stress Dual-energy Computed Tomography to Assess Myocardial PerfusionOnce scout views from the tracheal bifurcation to the diaphragm in the craniocaudal direction were taken and the scan was planned, adenosine was administered with a perfusion pump (Alaris System, Cardinal Health; Ohio, United States) at a constant rate of 140 μg/kg/min; 3 min afterward, 60mL of iopromide (Ultravist 370, Bayer Schering Pharma; Berlin, Germany) iodine contrast agent was administered, followed by 60mL of saline, at 4mL/s, with an injector (Stellant Dual, Medrad; Pennsylvania, United States). A retrospective electrocardiogram-gated dual-energy scan with tube current modulation was performed with the following technical characteristics: rotation time, 330ms; heart rate-dependent pitch, 0.2-0.43, collimation, 0.6mm; and temporal resolution, 165ms. The tubes operated with 165 mAs/rot at 100 kV and with 140 mAs/rot at 140kV. Tube current modulation was performed with the MinDose technique with full tube current applied between 60% and 75% of the cardiac cycle, which was reduced to 4% outside this time window.
To achieve adequate enhancement, bolus tracking was performed by placing a region of interest in the aortic arch with a trigger threshold of 160 HU. A delay of 10s was added to guarantee adequate myocardial perfusion.
Adenosine infusion was stopped once the acquisition was finished. Blood pressure, electrocardiogram, and clinical symptoms were carefully monitored.
A monosegment reconstruction algorithm that uses data from a complete rotation of both detectors was used for image reconstruction. Data were reconstructed in diastole from 60% to 75% of the R-R interval, with a 3-mm slice thickness, 1.5-mm reconstruction interval, and D30f reconstruction kernel.
Coronary Computed Tomography Angiography to Assess Coronary AnatomyAfter 5min, coronary CT angiography was performed. If there was no contraindication and the heart rate was >65 bpm, beta-blocker (5-15mg intravenous metoprolol) was administered. If the heart rate was stable and <65 bpm, prospective acquisition was performed with the high-pitch technique. If a stable heart rate of <65 bpm could not be achieved, retrospective electrocardiogram-gated spiral acquisition was performed. Sublingual nitroglycerine (0.5-1mg) was administered to all patients.
The technical parameters for the prospective high-pitch acquisition were as follows: collimation, 0.6mm (128×0.6 mm); rotation time, 280 ms; effective tube current, 370 mAs with CARE DOSE modulation; 100 or 120 Kv current, depending on body weight <80 or >80 kg, respectively; pitch, 3.4; and temporal resolution, 75ms. The acquisition was performed in the caudocranial direction beginning at 60% of the R-R interval.
The technical parameters for the retrospective acquisition were as follows: collimation, 0.6mm (128×0.6mm); rotation time, 280ms; 100 or 120 Kv current, depending on body weight <80 or >80kg, respectively; effective tube current, 370 mAs; heart rate-dependent pitch, 0.2-0.43; and MinDose tube current modulation with the maximum current of the tube between 60% and 75% of the cardiac cycle, reduced to 4% outside this time window.
Vascular enhancement was achieved by the administration of 60mL of iopromide (Ultravist 370, Bayer Schering Pharma), followed by 60mL of saline, at a constant rate of 6mL/s. Bolus tracking was performed by placing a region of interest in the aortic arch with a trigger threshold of 100 HU.
In all cases, image reconstruction was performed with a 0.6-mm slice thickness, 0.4mm, reconstruction interval, and B26f reconstruction kernel. When the acquisition was retrospective, the reconstruction was obtained in the phase of the cardiac cycle where the coronary arteries could best be assessed.
Significant stenosis was considered to be present when the stenosis size was >50% of the lumen diameter on quantitative analysis.
The myocardial images of this series were evaluated in resting perfusion.
Computed Tomography to Assess Myocardial ViabilityAfter 7min, a prospective acquisition was performed with the high-pitch technique, with the same settings as those of coronary CT angiography, except that a lower radiation dose was used with 80kV and 300 mAs/rot. No intravenous contrast agent or any other drug was administered.
Image reconstruction was performed with a 0.6-mm slice thickness, 0.4-mm reconstruction interval, and B26f reconstruction kernel.
Cardiac Magnetic Resonance Imaging ProtocolCardiac MR imaging was performed with a 1.5-T MR imaging system (Achieva, Philips Medical Systems; Eindohven, Netherlands) with a 5-element surface coil array specific for cardiac scans (SENSE Cardiac, Philips Medical Systems) and the following protocol:
First-Pass Stress PerfusionIntravenous adenosine was administered via a perfusion pump (Alaris System, Cardinal Health) at a rate of 140 μg/kg/min. Blood pressure, heart rate, and oxygen saturation were monitored at 1-min intervals. After 3min, a bolus of 0.05 mmol/kg gadobutrol (Bayer Schering Pharma) intravenous contrast agent was injected at 4mL/s with 40 mL of saline at 4 mL/s through the antecubital vein in the left arm. The first-pass perfusion was performed with a T1-weighted turbo gradient echo (TGE) sequence (repetition time, 2.2ms; echo time, 1.04ms; angle, 50°; slice thickness, 10mm) in the short-axis, which included the base, the middle third, and apex of the left ventricle. The images were acquired at end-diastole to maximize the intravascular signal.
First-Pass Rest PerfusionApproximately 5min after performing the stress perfusion, contrast was administered again and baseline perfusion was assessed with the same technique.
Myocardial ViabilityAfter a 10-min wait, the delayed enhancement images were acquired. Before the viability images were acquired, the optimal inversion time was calculated to best cancel out the myocardial signal with a TGE-echo planar sequence (repetition time, 40ms; echo time, 5ms; angle, 15°; slice thickness, 10mm). For the delayed enhancement, a T1-weighted 3-dimensional-TGE sequence with a tissue preparation pulse was used (repetition time, 3.9-4.3ms; echo time, 1.2-1.3 ms; angle, 15°; slice thickness, 10mm). All images were acquired during breath holding in the short- and long-axes of the left ventricle and with a 4-chamber view.
Image Postprocessing and InterpretationThe CT and MR imaging reconstructions were independently interpreted by 2 observers (with 8 and 10 years of cardiac imaging experience) for each of the 17 myocardial segments of the American Heart Association classification, without knowing the results of the other tests. Subsequently, the observers analyzed the images together to resolve any discrepancies and reach a consensus.
To evaluate the MPI of DECT, iodine maps in the cardiac short-axis, with a slice thickness of 5mm, were generated with Syngo Multimodality Workplace postprocessing software (Syngo Dual Energy, Siemens). Perfusion defects were defined as contiguous and circumscribed areas with a reduction or absence of iodine compared with that of normal myocardium of the left ventricle.15
To evaluate myocardial viability on CT, multiplanar reconstructions were performed on a Leonardo workstation (Siemens) in the short- and long-axes and the 4-chamber view, with a slice thickness of 10mm.
The comparison between CT and MR imaging with regard to the analysis of perfusion and myocardial viability used MR imaging as the reference standard.
Radiation DoseThe effective radiation dose for the CT scan was calculated by multiplying the dose-length product by the conversion factor for the chest (k=0.014 mSv/mGy×cm) according to the following formula: estimated effective dose (mSv)=dose-length product (mGy-cm)×0.014 (conversion factor for the chest; mSv/mGy×cm).16
Statistical AnalysisIn the descriptive statistical analysis, quantitative variables are expressed as the mean (standard deviation) and qualitative variables as frequencies or percentages.
With the cardiac MR imaging as a reference, the diagnostic accuracy of CT for the evaluation of myocardial perfusion defects and delayed enhancement was expressed in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
Qualitative variables were assessed with the χ2 test.
RESULTSStudy PopulationThe protocol was completed in 56 of the 68 referred patients; 12 patients were excluded because they did not consent to MR imaging.
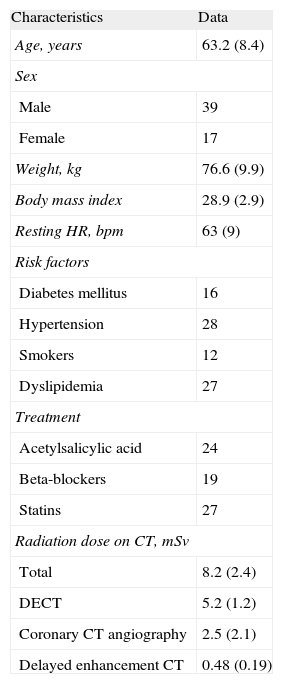
The clinical characteristics of the patients and the radiation doses are summarized in Table 1. The indication for the tests was chest pain in 51 patients and electrocardiogram changes in 5. One patient had undergone coronary revascularization surgery and 6 had undergone coronary stent implantation. The mean interval between CT and cardiac MR imaging was 10.8 (5.1) days.
Clinical Characteristics of the Patients
| Characteristics | Data |
| Age, years | 63.2 (8.4) |
| Sex | |
| Male | 39 |
| Female | 17 |
| Weight, kg | 76.6 (9.9) |
| Body mass index | 28.9 (2.9) |
| Resting HR, bpm | 63 (9) |
| Risk factors | |
| Diabetes mellitus | 16 |
| Hypertension | 28 |
| Smokers | 12 |
| Dyslipidemia | 27 |
| Treatment | |
| Acetylsalicylic acid | 24 |
| Beta-blockers | 19 |
| Statins | 27 |
| Radiation dose on CT, mSv | |
| Total | 8.2 (2.4) |
| DECT | 5.2 (1.2) |
| Coronary CT angiography | 2.5 (2.1) |
| Delayed enhancement CT | 0.48 (0.19) |
CT, computed tomography; DECT, dual-energy computed tomography; HR, heart rate.
Following adenosine administration, heart rate increased from 63.7 (9.3) bpm at rest to 75.5 (13.8) bpm. There were no major complications that hampered scan completion, although 9 patients had 2:1 atrioventricular blocks, 3 had chest discomfort, and 4 had headache. All these complications resolved after stopping adenosine infusion. The estimated effective dose was 5.2 (1.2) mSv. Perfusion defects were found in 17 of the 56 patients (30.3%), 29 of the 168 coronary artery territories (17.2%), and 55 of the 952 myocardial segments studied (5.7%).
Coronary Computed Tomography AngiographyWe performed high-pitch prospective acquisition (heart rate, 57 [6] bpm) in 44 patients and retrospective acquisition (heart rate, 74 [5] bpm) in 12. The estimated effective dose was 2.5 (2.1) mSv. In a per-patient analysis, 27 had normal coronary arteries or <50% stenosis, 15 had significant stenosis (single-vessel disease in 11, 2-vessel in 2, and 3-vessel in 3), and 14 had at least 1 artery that could not be assessed due to an artifact or severe calcification. In a per-artery analysis, the anterior descending, right coronary, and circumflex arteries were normal or without significant stenosis in 37, 34, and 37 patients, with significant stenosis in 5, 8, and 8 patients, and were impossible to assess in 14, 14, and 11 patients, respectively.
Computed Tomography for Myocardial ViabilityHyperenhancement was seen in 10 of 56 patients (17.8%), 14 of 168 coronary artery territories (8.3%), and 28 of 952 myocardial segments studied (2.9%). The estimated effective dose was 0.48 (0.19) mSv.
Cardiac Magnetic Resonance ImagingImages of a sufficient quality to assess perfusion defects and delayed enhancement were obtained in all 56 patients. Perfusion defects were found in 21 patients (37.5%), 33 coronary artery territories (19.6%), and 68 myocardial segments (7.1%). Delayed hyperenhancement was seen in 12 patients (21.4%), 18 coronary artery territories (10.7%), and 37 myocardial segments (3.8%).
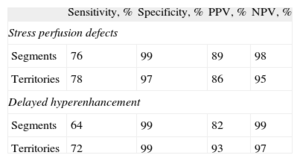
Comparison Between Myocardial Perfusion Imaging With Cardiac Computed Tomography and Cardiac Magnetic Resonance ImagingThe sensitivity, specificity, and negative and positive predictive values of cardiac CT for perfusion defects and delayed hyperenhancement are shown in Table 2.
Results of the Dual-source Computed Tomography Analysis of Perfusion Defects and Delayed Hyperenhancement
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
| Stress perfusion defects | ||||
| Segments | 76 | 99 | 89 | 98 |
| Territories | 78 | 97 | 86 | 95 |
| Delayed hyperenhancement | ||||
| Segments | 64 | 99 | 82 | 99 |
| Territories | 72 | 99 | 93 | 97 |
NPV, negative predictive value; PPV, positive predictive value.
Of the 62 myocardial segments with perfusion defects on DECT, 55 were found with first-pass perfusion on MR imaging (Fig. 1). However, the other 7 segments with perfusion defects were not identified on MR imaging, and 15 segments with perfusion changes on MR imaging were not seen on DECT perfusion analysis.
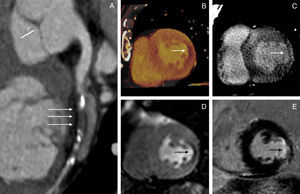
Patient showing concordance between computed tomography and magnetic resonance imaging. A 67-year-old man with significant stenosis in the circumflex artery (A) and a perfusion defect in the lateral wall of the left ventricle on computed tomography (B) and magnetic resonance imaging (D). Delayed enhancement was seen with both techniques (C and E).
Delayed hyperenhancement was found in 28 segments on CT, of which 23 coincided with those of MR imaging. There were 13 hyperenhanced segments on MR imaging that were not seen on CT (Fig. 2).
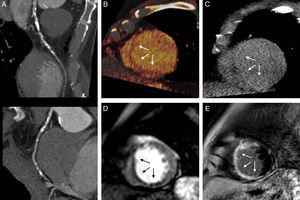
False negative in computed tomography assessment of the viability of diseased coronaries. A 72-year-old man with lesions in the anterior descending artery (A) and a perfusion defect in the septum on computed tomography (B) and magnetic resonance imaging (D). The delayed enhancement seen on magnetic resonance (E) was not seen on computed tomography (C).
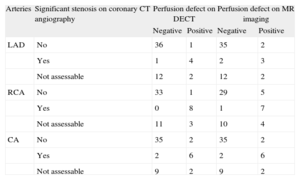
We found 21 coronary arteries with stenosis >50% and 107 without stenosis. Table 3 details the relationship between the coronary CT angiography findings and the presence of perfusion defects. Of the 14 patients with at least 1 nonassessable coronary artery, a perfusion defect was seen on CT in the corresponding territory in 3 patients.
Relationship Between Stenosis on Coronary Computed Tomography Angiography and Perfusion Defects on Both Dual-energy Computed Tomography and Magnetic Resonance Imaging
| Arteries | Significant stenosis on coronary CT angiography | Perfusion defect on DECT | Perfusion defect on MR imaging | ||
| Negative | Positive | Negative | Positive | ||
| LAD | No | 36 | 1 | 35 | 2 |
| Yes | 1 | 4 | 2 | 3 | |
| Not assessable | 12 | 2 | 12 | 2 | |
| RCA | No | 33 | 1 | 29 | 5 |
| Yes | 0 | 8 | 1 | 7 | |
| Not assessable | 11 | 3 | 10 | 4 | |
| CA | No | 35 | 2 | 35 | 2 |
| Yes | 2 | 6 | 2 | 6 | |
| Not assessable | 9 | 2 | 9 | 2 | |
CA, circumflex artery; CT, computed tomography; DECT, stress dual-energy computed tomography; LAD, left anterior descending artery; MR, stress magnetic resonance imaging; RCA, right coronary artery.
The current study indicates that DSCT is a viable technique that provides acceptable diagnostic results compared with those of MR imaging in the assessment of myocardial perfusion. Furthermore, CT scanning permits an image of the coronary arteries to be obtained that helps prognostic stratification of atherosclerosis.
Coronary CT angiography is an excellent technique for the evaluation of coronary artery disease; however, it does not provide information on myocardial perfusion, because coronary flow is maintained until the stenosis exceeds 80% of lumen diameter.17 Accordingly, when coronary artery disease is diagnosed, the effect on myocardial perfusion can often only be assessed though stress testing.
The technological development of CT scanners has allowed good quality perfusion studies to be performed with an appropriate radiation dose. Blankstein et al.18 have reported that stress MPI studies with DSCT have a sensitivity of 93% and a specificity of 74% for coronary artery stenosis detection in comparison with SPECT. Rocha-Filho et al.19 found that the addition of MPI increased the diagnostic accuracy of coronary CT angiography, thereby enabling the simultaneous evaluation of anatomy and perfusion.
Recently, Bettencourt et al.20 demonstrated the high sensitivity and specificity of MPI for both CT and MR imaging in the detection of significant coronary stenoses in a study that used the fractional flow reserve as a reference. The authors showed that a CT protocol that integrates anatomy and perfusion can be as effective as MR perfusion imaging for the detection of significant coronary artery lesions.
Another more novel method for performing MPI is DECT, through which an iodine map can be generated that reflects the distribution of this contrast agent in the myocardium and which is based on the specific absorption characteristics of X-rays by iodine at high and low energy levels.21 Ko et al.12,13 reported that DECT is safe for evaluating myocardial perfusion defects compared with MR imaging. Furthermore, they demonstrated that studies with iodine perfusion maps increase the diagnostic accuracy of coronary CT angiography in patients with known coronary artery disease.
CT can also assess delayed enhancement because iodine is an extracellular contrast agent that persists in the presence of myocardial necrosis. High-pitch DSCT has been shown to be accurate in the evaluation of delayed enhancement with a low radiation dose, although the images are more “noisy”.14
Here, a novel protocol with a second-generation DSCT system was used to evaluate myocardial perfusion with dual-energy mode and an iodine map, and coronary arteries and delayed enhancement with the high-pitch technique. In contrast to other studies, we included participants with clinical suspicion of coronary artery disease in the cardiology clinic, without an imaging test that revealed the presence of coronary artery disease. In fact, the prevalence of coronary artery disease in our study population was relatively low. The values of our study in the comparison between MPI with an iodine map and MR imaging are similar to those of Ko et al.,12 who performed a similar comparison, but in patients with known coronary artery disease. However, the sensitivity of DECT for the detection of delayed hyperenhancement was only 64%, which was probably affected by the low kilovoltage used and which resulted in the acquisition of “noisy” images.
We also studied the anatomy of the coronary arteries, and found an excellent correlation between coronary artery stenosis and perfusion defects, both on DECT and MR imaging.
In our study, 3 patients with nonassessable coronary arteries showed perfusion changes on CT. These patients were treated differently and were referred for coronary angiography.
LimitationsOur study has several limitations. First, the study was performed in a single center. Accordingly, random prospective multicenter studies are required to evaluate the use of this protocol in the study of ischemic heart disease.
MPI with an iodine map is a recently introduced technique and it remains to be documented how to obtain an optimal image (eg, administration rate and quantity of contrast agent, the initiating time of the acquisition, technical characteristics). Furthermore, beam hardening artifacts are sometimes observed that can cause false positives. The technique also needs to be learned in order to best set up the scan and improve image interpretation.
Another problem is that the iodine perfusion map is not dynamic: only the distribution of the iodine at a particular moment in time is assessed, which may not be the optimal moment to show the attenuation difference between poorly perfused and normal myocardium. The static image also lacks quantitative perfusion data, such as arterial blood flow and volume.
We used only MR imaging as a reference guide, without the use of invasive studies such as coronary angiography to assess coronary artery stenosis and coronary flow reserve.
Finally, the radiation dose of our protocol was still quite high (8.2 [2.4] mSv), although it was nonetheless lower than that of SPECT perfusion scans (12.3 [4.3] mSv).22
Implications for Clinical PracticeDespite all these limitations, DSCT, in the absence of contraindications, is a diagnostic technique that offers acceptable results for the evaluation of myocardial ischemia. This method can simultaneously aid in the assessment of functional effects in cases of coronary stenosis that are difficult to quantify due to, for example, significant calcium deposition. This imaging modality would allow a more precise identification of patients suitable for coronary angiography due to MPI alterations.
CONCLUSIONSDSCT is a promising technique for the evaluation of perfusion defects and myocardial viability that allows the concomitant assessment of coronary anatomy with an acceptable radiation dose. Larger multicenter studies are needed to determine its true value.
CONFLICTS OF INTERESTNone declared.