Myocardial strain analysis could provide additional information to left ventricular ejection fraction (LVEF) in nonischemic dilated cardiomyopathy (NIDC). Our aim was to analyze the feasibility of left ventricular strain evaluation using cardiac magnetic resonance feature tracking (FT) in NIDC, and to determine its clinical and prognostic impact.
MethodsWe retrospectively included consecutive patients with NIDC who underwent cardiac magnetic resonance. Left ventricular global longitudinal, circumferential and radial strain were obtained from standard cine sequences using FT analysis software. We evaluated their association with a composite endpoint (heart failure, implantable cardioverter-defibrillator in secondary prevention, or death).
ResultsFT analysis could be performed in all of the 98 patients (mean age 68±13 years, 72% men). Intra- and interobserver concordance was good for global longitudinal and circumferential strain but was worse for radial strain. Global circumferential strain was independently associated (OR, 1.16; P=.045) with LVEF normalization during follow-up and was the only morphological parameter independently associated with the composite endpoint (OR, 1.15; P=.038). A cutoff value <−8.2% was able to predict the incidence of this event during follow-up (log-rank 4.6; P=.032).
ConclusionsLeft ventricular strain analysis with FT is feasible and reproducible in NIDC. Global circumferential strain was able to predict LVEF recovery and the appearance of major cardiovascular events during follow-up.
Keywords
Nonischemic dilated cardiomyopathy (NIDCM) is associated with elevated mortality during follow-up,1 despite recent advances in its medical therapy. Unfortunately, NIDCM risk cannot be adequately stratified using left ventricular ejection fraction (LVEF).2 In this context, cardiac magnetic resonance (CMR) is particularly useful, given that late gadolinium enhancement (LGE) supports the etiological diagnosis and predicts adverse events independently of LVEF.3 In addition, myocardial strain analysis using speckle tracking echocardiography has additive value for risk stratification in NIDCM.4 However, despite being the most widely used technique, it has some limitations.5 An analogous tool has recently been developed for CMR, called feature tracking (FT),6 which permits evaluation of strain via standard cine sequences with a simple postprocessing method. Normal values are already available for this technique7 and its feasibility has been proven in different situations.8 In the case of NIDCM, recent studies have obtained contradictory results on the FT-derived parameter with greatest clinical use and its prognostic impact.9,10
The aim of the present study was to analyze the feasibility of left ventricular (LV) myocardial strain evaluation using FT in NIDCM. An additional objective was to evaluate the prognostic impact of LV global longitudinal strain (GLS), global circumferential strain (GCS), and global radial strain (GRS).
METHODSStudy populationThe present study retrospectively analyzed patients who underwent CMR in our center between February 2011 and March 2017 with a final diagnosis of NIDCM, defined according to current recommendations.11 The patients’ baseline characteristics and electrocardiogram and treatment data were collected from their medical records. Changes over time in the main morphofunctional variables were studied in the patients who underwent follow-up echocardiography. Similarly, the incidences were analyzed of other variables, such as heart failure, implantable cardioverter-defibrillator (ICD) implantation, and death. In addition, a composite endpoint was examined: hospitalization due to heart failure, ICD implantation in secondary prevention, and overall death.
Acquisition and analysis of cardiac magnetic resonance studiesCMR studies were performed using a 1.5-T Signa HDxt system (GE Healthcare, United States); standard SSFP cine sequences were obtained (20 images/cycle) in longitudinal axes and in 10 to 15 contiguous short-axis slices covering both ventricles from the base to the apex. LGE images were acquired about 8 to 10minutes after intravenous infusion of gadobutrol at 0.2 mmol/kg (gadovist 1 mmol/mL). The volumes and ejection fractions of both ventricles were calculated from the cine sequences using the disk summation method with specific software (ReportCARD, GE Healthcare, United States). Hyperenhancement presence and distribution were qualitatively evaluated in the LGE sequences.
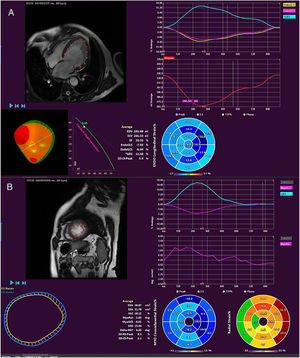
The FT analysis was performed using QStrain RE v2.0 software (Medis, The Netherlands). Using short- and long-axis views, end-diastolic and endsystolic volumes were obtained, as well as ejection fraction and the LV myocardial strain parameters: GLS, GCS, and GRS (figure 1). To determine intraobserver variability, the same researcher later reanalyzed 20 randomly chosen patients. The interobserver reproducibility was assessed through the analysis of 20 randomly chosen patients by an experienced CMR reader.
Determination of myocardial strain using feature tracking. In these longitudinal projections (A), the global longitudinal and circumferential strain can be determined and the radial strain inferred, as well volumes and ejection fractions, whereas, in the short axis (B), the global circumferential and radial strain can be measured and data obtained on myocardial torsion.
Qualitative variables are presented as absolute number and percentage and quantitative variables as mean ± standard deviation or median [interquartile range] as appropriate. The intraclass correlation coefficient (ICC) was used to evaluate the intraobserver and interobserver variabilities in the FT determination of strain. Similarly, the Pearson r coefficient and ICC were used to evaluate the concordance between the measurements obtained using the traditional disk summation method and the FT method. The chi-square test was used to compare categorical variables. Differences in continuous variables with normal and nonnormal distributions were evaluated using the unpaired Student t test and the Mann-Whitney U test, respectively. The association was analyzed of the FT-derived LV strain parameters with event occurrence. To identify independent prognostic factors, logistic regression models were built using backward selection with other morphofunctional CMR variables. The results are expressed as odds ratios (ORs) with 95% confidence intervals (95%CIs). ROC curves were used to identify the strain values with best predictive ability. These were used in the Kaplan-Meier survival curve analysis and, together with other morphofunctional CMR variables, using Cox regression with backward selection. Differences were considered statistically significant at a 2-tailed P value < .05. The data analysis was performed with SPSS version 18.0 (SPSS Inc, United States).
RESULTSPopulation characteristicsIn total, 98 patients diagnosed with NIDCM were finally included and FT analysis could be performed in all patients. Significant coronary heart disease was ruled out using invasive coronary angiography (89%) or cardiac computed tomography (4.1%). No patients had LGE with an ischemic pattern.
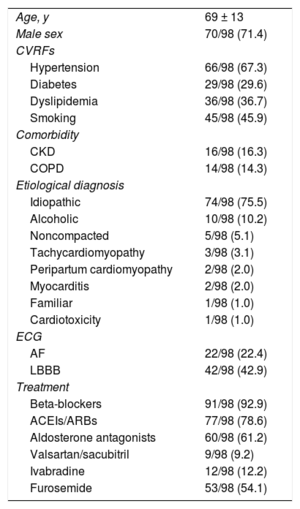
Regarding the baseline characteristics (table 1), 71.4% were men and the mean age was 69 ± 13 years. The cardiomyopathy was considered idiopathic in 75.5% and alcoholic in 10.2%. Relevant findings in the pre-CMR electrocardiogram included atrial fibrillation in 22% of patients and complete left bundle branch block in 42.9%. A high level of optimal medical therapy was seen (including beta-blockers, 92.8%; angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin II receptor blockers [ARBs], 78.5%; and aldosterone antagonists, 61%).
Baseline characteristics
| Age, y | 69 ± 13 |
| Male sex | 70/98 (71.4) |
| CVRFs | |
| Hypertension | 66/98 (67.3) |
| Diabetes | 29/98 (29.6) |
| Dyslipidemia | 36/98 (36.7) |
| Smoking | 45/98 (45.9) |
| Comorbidity | |
| CKD | 16/98 (16.3) |
| COPD | 14/98 (14.3) |
| Etiological diagnosis | |
| Idiopathic | 74/98 (75.5) |
| Alcoholic | 10/98 (10.2) |
| Noncompacted | 5/98 (5.1) |
| Tachycardiomyopathy | 3/98 (3.1) |
| Peripartum cardiomyopathy | 2/98 (2.0) |
| Myocarditis | 2/98 (2.0) |
| Familiar | 1/98 (1.0) |
| Cardiotoxicity | 1/98 (1.0) |
| ECG | |
| AF | 22/98 (22.4) |
| LBBB | 42/98 (42.9) |
| Treatment | |
| Beta-blockers | 91/98 (92.9) |
| ACEIs/ARBs | 77/98 (78.6) |
| Aldosterone antagonists | 60/98 (61.2) |
| Valsartan/sacubitril | 9/98 (9.2) |
| Ivabradine | 12/98 (12.2) |
| Furosemide | 53/98 (54.1) |
ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVRFs, cardiovascular risk factors; ECG, electrocardiography; LBBB, complete left bundle branch block.
Values represent No. (%) or mean ± standard deviation.
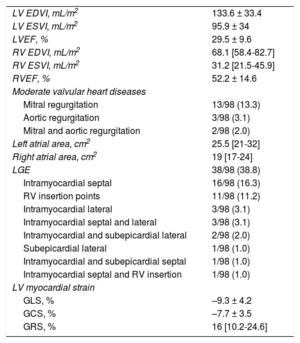
The morphological and functional characteristics of CMR are collected in table 2. The study patients had severe LV dilatation (end-diastolic volume index [EDVI], 133.6 ± 33.4mL/m2) with severe systolic dysfunction (LVEF, 29.5% ± 9.6%). However, most patients had a right ventricle of normal size and function. In 38.8%, LGE revealed the presence of fibrosis; the most frequent pattern was intramyocardial septal (16.3%). In line with the above LV function values, the myocardial strain parameters were significantly altered: GLS, –9.3% ± 4.2%; GCS, –7.7% ± 3.5%; and GRS, 16% [10.2%-24.6%]. Evaluation of the associations among the variables showed a good correlation of GLS with GCS (r = 0.739; P < .001) and a moderate correlation of GLS with GRS (r = –0.539; P < .001).
Morphological and functional cardiac magnetic resonance characteristics
| LV EDVI, mL/m2 | 133.6 ± 33.4 |
| LV ESVI, mL/m2 | 95.9 ± 34 |
| LVEF, % | 29.5 ± 9.6 |
| RV EDVI, mL/m2 | 68.1 [58.4-82.7] |
| RV ESVI, mL/m2 | 31.2 [21.5-45.9] |
| RVEF, % | 52.2 ± 14.6 |
| Moderate valvular heart diseases | |
| Mitral regurgitation | 13/98 (13.3) |
| Aortic regurgitation | 3/98 (3.1) |
| Mitral and aortic regurgitation | 2/98 (2.0) |
| Left atrial area, cm2 | 25.5 [21-32] |
| Right atrial area, cm2 | 19 [17-24] |
| LGE | 38/98 (38.8) |
| Intramyocardial septal | 16/98 (16.3) |
| RV insertion points | 11/98 (11.2) |
| Intramyocardial lateral | 3/98 (3.1) |
| Intramyocardial septal and lateral | 3/98 (3.1) |
| Intramyocardial and subepicardial lateral | 2/98 (2.0) |
| Subepicardial lateral | 1/98 (1.0) |
| Intramyocardial and subepicardial septal | 1/98 (1.0) |
| Intramyocardial septal and RV insertion | 1/98 (1.0) |
| LV myocardial strain | |
| GLS, % | –9.3 ± 4.2 |
| GCS, % | –7.7 ± 3.5 |
| GRS, % | 16 [10.2-24.6] |
EDVI, end-diastolic volume index; ESVI, endsystolic volume index; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricular; RVEF, right ventricular ejection fraction.
Values represent No. (%), mean ± standard deviation, or median [interquartile range].
In terms of the intraobserver variability, there was an excellent ICC for GLS (0.95; P < .001) and GCS (0.87; P < .001) and a good ICC for GRS (0.62; P = .021). Similar, although slightly worse, concordance values were found for the interobserver evaluation: GLS (0.98; P < .001), GCS (0.56; P = .042), and GRS (0.45; P = .103). The time required for myocardial strain analysis using FT was 7.8 ± 2.3minutes.
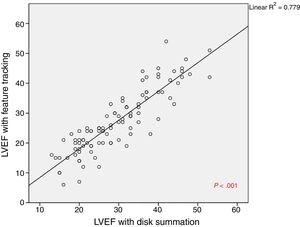
In addition, a comparison of the LV volume and function values determined by the traditional disk summation method and by the FT method revealed high concordance between the 2 techniques: EDVI (r = 0.896, P < .001; ICC = 0.838, P < .001), endsystolic volume index (r = 0.944, P < .001; ICC = 0.928, P < .001), and LVEF (r = 0.882, P < .001; ICC = 0.923, P < .001) (figure 2).
Correlation of myocardial strain values with baseline and morphofunctional characteristicsNo associations were found between the baseline clinical characteristics and the FT-derived LV myocardial strain values, except for a weak negative correlation between age and GRS (r = –0.28; P = .005). Similarly, there were no differences in the values obtained according to the presence of atrial fibrillation or complete left bundle branch block during the test.
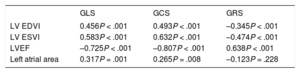
Both LV volumes and functions and left atrial size were significantly correlated with all LV strain values obtained using FT (table 3), particularly GCS. However, no differences were seen in the values according to the presence of fibrosis on LGE.
Correlation of feature tracking-determined myocardial strain values with morphological and functional cardiac magnetic resonance variables
| GLS | GCS | GRS | |
|---|---|---|---|
| LV EDVI | 0.456P < .001 | 0.493P < .001 | –0.345P < .001 |
| LV ESVI | 0.583P < .001 | 0.632P < .001 | –0.474P < .001 |
| LVEF | –0.725P < .001 | –0.807P < .001 | 0.638P < .001 |
| Left atrial area | 0.317P = .001 | 0.265P = .008 | –0.123P = .228 |
EDVI, end-diastolic volume index; ESVI, endsystolic volume index; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LV, left ventricle; LVEF, left ventricular ejection fraction.
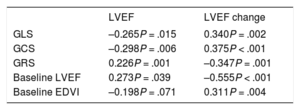
Follow-up echocardiography was performed in 84 of the patients (85.7%) at a median of 2.4 [1.8-3.4] years after the CMR study. These patients had an LVEF of 43.6% ± 10.8%, with at least moderate mitral regurgitation in 20 patients (20.4%) and an E/e’ value of 10 [7-14]. In addition, 71.4% had a higher LVEF in this study than in the baseline CMR, with a median increase of 13%; a LVEF > 50% was found in 25 patients (25.5%). Both the LVEF during follow-up and the change from baseline were significantly correlated with all FT-derived LV values and with the baseline LVEF on CMR (table 4). In contrast, the presence of LGE was only associated with a lower LVEF during follow-up (40.4% vs 45.5%; P = .04). Those patients with preserved systolic function (LVEF > 50%) during follow-up had been found to have better GCS values (–9% vs –7.1%; P = .019), without differences in the other FT-derived LV values or the baseline LVEF or EDVI. LGE was less frequent in these patients (16.1% vs 37.7%; P = .037). On multivariate analysis, GCS was independently associated with the presence of an LVEF > 50% during follow-up (OR = 1.16; 95%CI, 1-1.34; P = .045), and not LGE.
Correlation of feature tracking-determined myocardial strain values and baseline ventricular volume and function with systolic function on follow-up echocardiography
| LVEF | LVEF change | |
|---|---|---|
| GLS | –0.265P = .015 | 0.340P = .002 |
| GCS | –0.298P = .006 | 0.375P < .001 |
| GRS | 0.226P = .001 | –0.347P = .001 |
| Baseline LVEF | 0.273P = .039 | –0.555P < .001 |
| Baseline EDVI | –0.198P = .071 | 0.311P = .004 |
EDVI, end-diastolic volume index; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LVEF, left ventricular ejection fraction.
Event incidence during a median follow-up of 3.2 [2.2-4] years is shown in table 5. An ICD was implanted in 22 patients, mainly in primary prevention. Of the patients with an ICD indication in secondary prevention, 2 had sustained monomorphic ventricular tachycardia, 2 had syncope and nonsustained monomorphic ventricular tachycardia, and 1 had cardiac arrest. In total, 25.5% of patients required admission due to HF. Ten patients died during follow-up, with a cardiovascular cause confirmed in 5 of them. Of the remaining deaths, 4 patients died at home less than 24hours after symptom onset, with an unidentified immediate cause of death, and 1 patient died of respiratory failure of unknown cause.
Cardiovascular events during follow-up
| De novoAF | 6 (6.1%) |
| Intracardiac devices | |
| ICDs | 22 (22.4%) |
| Primary prevention | 17 (17.3%) |
| Secondary prevention | 5 (5.1%) |
| CRT | 13 (13.3%) |
| Pacemakers | 1 (1%) |
| Admissions due to HF | 25 (25.5%) |
| Total mortality | 10 (10.2%) |
| Cardiovascular cause | 5 (5.1%) |
| Unknown etiology | 5 (5.1%) |
| Composite event (HF/death/ICD in secondary prevention) | 34 (34.7%) |
AF, atrial fibrillation; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator.
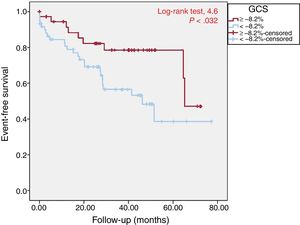
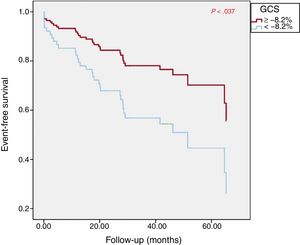
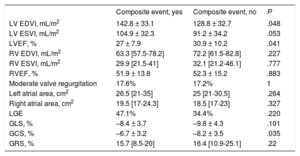
No significant association was found between admission due to HF and FT-derived LV myocardial strain values, except a tendency for lower GCS values (–7.1% vs –8.5%; P = .10). Mortality during follow-up was significantly associated with GCS (–5.9% vs –7.9%; P = .012) and tended to be associated with GLS (–6.9% vs –9.6%; P = .051). There were no differences in FT-derived strain values according to ICD implantation in secondary prevention. During follow-up, 34 patients (34.7%) had the composite event (admission due to HF, ICD in secondary prevention, or overall mortality); of the FT-derived strain parameters, only GCS was associated with this outcome (table 6). Of the remaining baseline morphological variables on CMR, only LVEF and EDVI were significantly associated with worse prognosis. Accordingly, the multivariate analysis included GCS, LVEF, and EDVI. GCS was independently associated with prognosis (OR = 1.15; 95%CI, 1.01-1.31; P = .038). Through the ROC curve, a GCS value of –8.2% was selected because it showed the best predictive performance (sensitivity, 74%; specificity, 45%). The Kaplan-Meier curve showed that this value was able to identify patients who had the composite event during follow-up (figure 3). This association was confirmed to be independent of other morphofunctional CMR variables with prognostic value (LVEF and EDVI) through Cox regression (figure 4), and a GCS < –8.2% protected against events during follow-up (OR = 0.44; 95%CI, 0.20-0.95; P = .037).
Association of the different morphofunctional cardiac magnetic resonance parameters with the composite prognostic event
| Composite event, yes | Composite event, no | P | |
|---|---|---|---|
| LV EDVI, mL/m2 | 142.8 ± 33.1 | 128.8 ± 32.7 | .048 |
| LV ESVI, mL/m2 | 104.9 ± 32.3 | 91.2 ± 34.2 | .053 |
| LVEF, % | 27 ± 7.9 | 30.9 ± 10.2 | .041 |
| RV EDVI, mL/m2 | 63.3 [57.5-78.2] | 72.2 [61.5-82.8] | .227 |
| RV ESVI, mL/m2 | 29.9 [21.5-41] | 32.1 [21.2-46.1] | .777 |
| RVEF, % | 51.9 ± 13.8 | 52.3 ± 15.2 | .883 |
| Moderate valve regurgitation | 17.6% | 17.2% | 1 |
| Left atrial area, cm2 | 26.5 [21-35] | 25 [21-30.5] | .264 |
| Right atrial area, cm2 | 19.5 [17-24.3] | 18.5 [17-23] | .327 |
| LGE | 47.1% | 34.4% | .220 |
| GLS, % | –8.4 ± 3.7 | –9.8 ± 4.3 | .101 |
| GCS, % | –6.7 ± 3.2 | –8.2 ± 3.5 | .035 |
| GRS, % | 15.7 [8.5-20] | 16.4 [10.9-25.1] | .22 |
EDVI, end-diastolic volume index; ESVI, endsystolic volume index; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; RVED, right ventricular ejection fraction.
Unless otherwise indicated, the data represent mean ± standard deviation or median [interquartile range].
Kaplan-Meier curve for composite event-free survival. A GCS value of –8.2% was able to discriminate patients who had heart failure, implantable cardioverter-defibrillator implantation in secondary prevention, or death during follow-up in the univariate analysis. GCS, global circumferential strain.
Cox regression curve for composite event-free survival. A GCS value of –8.2% was an independent predictor of the occurrence of heart failure, implantable cardioverter-defibrillator implantation, or death during follow-up, after adjustment of the survival curves for other morphofunctional CMR variables with prognostic relevance (LVEF and EDVI). CMR, cardiac magnetic resonance; EDVI, end-diastolic volume index; GCS, global circumferential strain; LVEF, left ventricular ejection fraction.
In our study, FT-derived LV strain values, particularly GCS, were not only correlated with morphofunctional markers of NIDCM severity, but could also predict events. First, GCS was found to be able to predict LVEF normalization, independently of LGE. Similar findings have been described for echocardiography-derived GLS in cardiotoxicity12 and could explain the association of strain parameters with interstitial fibrosis, which would determine systolic function recovery.13 As the most relevant finding, only GCS was independently associated with the composite event, and not the other morphofunctional CMR predictors (LVEF and EDVI). In addition, a GCS value < –8.2% protected against event occurrence during follow-up. Surprisingly, in contrast to other work,3 LGE was not associated with prognosis in our results. Some differences in patients’ characteristics could explain this discordance. For example, even though our population had worse systolic function at diagnosis, the prevalence of LGE in general, and its septal localization in particular, was lower. In turn, this could explain the lower incidence of events and the high rate of LVEF normalization during follow-up in our series. Finally, more recent work14 indicates that the predictive value of fibrosis is not specifically determined by its presence, but by its extent and distribution. In addition, previous studies corroborate the prognostic value of CMR strain in diverse clinical settings.
The usefulness of FT for NIDCM has recently been specifically evaluated. Romano et al.10 showed that FT-derived GLS provides additional information to LVEF and LGE in predicting mortality in a large population with ischemic and nonischemic dilated cardiomyopathy. However, the authors did not evaluate GCS or GRS. Specifically in NIDCM, Buss et al.9 found that not only was GLS associated with a higher rate of events during follow-up, but also GCS and GRS. Moreover, GLS improved the prognostic ability through its addition to EDVI, in conjunction with LVEF and LGE. Nonetheless, both the GCS and GRS were also associated with events in the multivariate analysis in the present study and, although the former had an interobserver variability similar to that of GLS, it was not considered in the final additive model.
Some factors could explain why GCS alone was independently associated with event occurrence in our study. First, in agreement with previous studies,15 GCS was found to be the FT parameter with highest reproducibility because it was not affected by poor tracking of the subannular region, unlike GLS. For this reason, it was considered the most robust parameter in FT studies of myocardial strain.5,15 In addition, our population had more advanced disease at diagnosis, reflected by the lower LVEF values and worse myocardial strain parameters. In this state, systolic function predominantly depends on the circumferential and radial contraction,16 and its alteration could influence prognosis, as seen in our series. In addition, given that epicardial fibers are the main contributors to the circumferential deformation, intramyocardial or subepicardial fibrosis, typical of NIDCM, may preferentially affect the GCS and permit better preservation of the other parameters.17
LimitationsFirst, despite the inherent limitation of a retrospective study in terms of the identification of events, their incidence was similar to that of other prospective studies.1,9 In addition, the small number of events only allowed the introduction of imaging variables in the multivariate analysis. Although this could limit the prognostic value of FT-derived strain from a more general context, it did allow demonstration of its superiority to other morphological predictors such as LVEF. Furthermore, our population had severe systolic dysfunction at diagnosis, which is why our findings might not be applicable to patients with less advanced disease. Finally, consideration is required of FT-related limitations. Compared with echocardiographic studies, FT has lower temporal and spatial resolution, although it does not suffer from acoustic window problems. In this regard, a standard SSFP sequence was used, with a temporal resolution of 20 images/cycle, reaching the minimum recommended for clinical studies of 45ms/phase.18 Although a higher temporal resolution could optimize the evaluation of myocardial strain, our work shows that, even with this resolution, common in clinical studies and used previously,10 FT has prognostic utility in NIDCM. In addition, although the GCS values are more reproducible, there is variability among the different software programs used for FT,19 which is why the reported cutoff points are not applicable to other tools.
CONCLUSIONSThe study of LV myocardial strain with FT using standard CMR sequences is fast, feasible, and highly reproducible in patients with NIDCM. In these patients, GCS may be an independent prognostic factor able to predict LVEF recovery and the occurrence of major cardiovascular events. Thus, CMR-FT analysis is potentially clinically useful in NIDCM.
CONFLICTS OF INTERESTNone to declare.
- –
Analysis of LV myocardial strain using FT is feasible and useful in diverse clinical situations and initial data support its prognostic value in NIDCM. However, previous studies had some limitations: they jointly evaluated patients with ischemic and nonischemic dilated cardiomyopathy, the patients had less advanced phases of the disease, and not all LV strain parameters were considered in the same manner.
- –
The results of this study confirm that it is possible to determine LV strain in all patients with NIDCM, even in the presence of severe dysfunction, with good reproducibility. In this clinical context, GCS was revealed to be the parameter with the best clinical usefulness, even better than that of GLS, given that it enabled the identification of patients with LVEF recovery during follow-up and was independently associated with major cardiovascular event occurrence during the clinical course.
The authors thank the technical and nursing staff and, by extension, the entire Chest Radiology Section of Hospital Clinic San Carlos for their ceaseless efforts to perform first-rate cardiac magnetic resonance studies.