Neprilysin has become a focus of interest in cardiology, due to the impressive benefits of combining neprilysin inhibition and angiotensin receptor blockade demonstrated in the recent PARADIGM-HF trial, which tested LCZ696 (now known as sacubitril/valsartan and marketed by Novartis under the name of Entresto) for treating systolic heart failure with reduced ejection fraction (HFrEF).1 However, neprilysin EC 3.4.24.11 (also known as neutral endopeptidase, endoprotease 24.11, NEP, common acute lymphoblastic leukemia antigen [CALLA], neutrophil antigen cluster differentiation antigen 10 [CD10], membrane metalloendopeptidase EC 3.4.24.11, and enkephalinase) is a highly versatile enzyme, which has returned to the spotlight, after an eventful career of > 40 years.2
In the cardiovascular system, neprilysin cleaves numerous vasoactive peptides. Some of these peptides have vasodilating effects (including natriuretic peptides, adrenomedullin, and bradykinin), and others have vasoconstrictor effects (angiotensin I and II, and endothelin-1, among others). Nevertheless, neprilysin displays various relative affinities among different substrates; its highest affinity is for atrial natriuretic peptide, C-type natriuretic peptide, and angiotensins I and II; its lowest affinity is for B-type natriuretic peptide (BNP), endothelin-1, and bradykinin.3
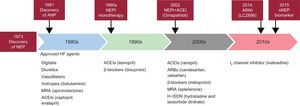
For decades, neprilysin has been an important biotarget. Academia and industry have combined active efforts to search for neprilysin inhibitors (NEPIs) that might be useful in clinical practice. Initially, in the late 1980s and early 1990s, NEPI monotherapy was tested. Candoxatril showed promising preliminary effects on hemodynamic parameters. However, another NEPI, ecadotril, led to higher mortality, with no evidence of clinical efficacy compared with placebo in patients with heart failure.4,5 Consequently, the development of NEPI monotherapy for heart failure was discontinued. Subsequently, some studies showed evidence of concurrent activation of the renin-angiotensin-aldosterone system, together with augmentation of natriuretic peptide bioactivity. These findings inspired the development and testing of agents that combined NEPI and angiotensin-converting enzyme inhibiting (ACEI) activity, which led to the drugs known as vasopeptidase inhibitors. Several vasopeptidase inhibitors have been developed, including omapatrilat, fasidotril, sampatrilat, and mixanpril. After numerous studies, the field was highly disappointed to find that omapatrilat provoked an increasing number of clinically relevant episodes of angioedema.6 After over a decade of wandering in the desert, a new concept was developed, the combination of NEPI and angiotensin II receptor blockers (ARBs), which led to a new class of drugs called angiotensin receptor neprilysin inhibitors. Sacubitril/valsartan is a first-in-class angiotensin receptor neprilysin inhibitor, which has shown better–than–expected results in the PARADIGM-HF trial1 (Figure).
Schematic of historical neprilysin highlights. ACEI, angiotensin-converting enzyme inhibitor; ANP, atrial natriuretic peptide; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; HF, heart failure; MRA, mineralocorticoid receptor antagonist; NEP, neprilysin; NEPI, neprilysin inhibitor; sNEP, soluble neprilysin.
PARADIGM-HF was a multinational, randomized, double-blind study of 8442 patients. The aim was to compare sacubitril/valsartan with enalapril in adult patients with chronic heart failure (New York Heart Association [NYHA] class II-IV) and reduced left ventricular ejection fraction (LVEF ≤ 40%, later amended to ≤ 35%), in addition to other heart failure therapy.1 The primary endpoint was the composite of cardiovascular death or hospitalization for heart failure. Prior to study participation, patients were treated with the standard of care therapy, which included ACEI/ARBs (> 99%), beta-blockers (94%), mineralocorticoid antagonists (58%), and diuretics (82%). The median follow-up duration was 27 months, and patients were treated for up to 4.3 years.
Patients were required to discontinue their existing ACEI or ARB therapy and enter a sequential, single-blind, run-in period. During the run-in period, they received treatment with enalapril 10mg twice daily, followed by a single-blind treatment with sacubitril/valsartan 100mg twice daily, which was increased to 200mg twice daily. They were then randomized to the double-blind period of the study. During that period, they received either sacubitril/valsartan 200mg or enalapril 10mg, twice daily. The mean age of the population studied was 64 years, and 19% were 75 years or older. At randomization, 70% of patients were NYHA class II, 24% were class III, and 0.7% were class IV. The mean LVEF was 29%, and there were 963 (11.4%) patients with a baseline LVEF > 35% and ≤ 40%. The study was prematurely terminated, due to the overwhelming reductions in death from cardiovascular causes and the reduction in the composite primary endpoint (cardiovascular death or hospitalization secondary to heart failure). The PARADIGM-HF trial is also referred to as the 20% trial, due to the homogeneous ∼20% relative reductions in all studied endpoints, including the composite primary endpoint of cardiovascular death, sudden cardiac death, and hospitalization for heart failure (Table 1).
PARADIGM-HF Trial: Treatment Effects on the Primary Composite Endpoint, Its Components, and All-cause Mortality, Over a Median Follow-up of 27 Months
| Endpoints | Hazard ratio (95%CI) | Relative risk reduction | P |
|---|---|---|---|
| Primary composite endpoint of CV death and hospitalizations due to heart failure | 0.80 (0.73–0.87) | 20% | .0000002 |
| Individual components of the primary composite endpoint | |||
| CV death | 0.80 (0.71–0.89) | 20% | .00004 |
| First heart failure hospitalization | 0.79 (0.71–0.89) | 21% | .00004 |
| Secondary endpoint | |||
| All-cause mortality | 0.84 (0.76–0.93) | 16% | .0005 |
95%CI, 95% confidence interval; CV, cardiovascular-related.
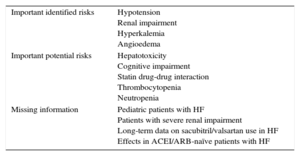
Although sacubitril/valsartan has shown enormous promise, there are challenges and unaddressed issues that merit additional studies and further clarification (Table 2). Some of these issues were raised and discussed in the European Medicines Agency assessment report on Entresto.7
Summary of Safety Concerns for Sacubitril/Valsartan
| Important identified risks | Hypotension Renal impairment Hyperkalemia Angioedema |
| Important potential risks | Hepatotoxicity Cognitive impairment Statin drug-drug interaction Thrombocytopenia Neutropenia |
| Missing information | Pediatric patients with HF Patients with severe renal impairment Long-term data on sacubitril/valsartan use in HF Effects in ACEI/ARB-naïve patients with HF |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HF, heart failure.
Modified from the European Medicines Agency Assessment Report.7
First, when patients experience tolerability issues (eg, systolic blood pressure [SBP] ≤ 95mmHg, symptomatic hypotension, hyperkalemia, renal dysfunction), current recommendations are to adjust concomitant drugs, and/or to down-titrate or discontinue sacubitril/valsartan temporarily. In fact, the European Medicines Agency recommends that treatment should not be initiated in patients with serum potassium levels > 5.4 mmol/L or with systolic blood pressure < 100mmHg.
Second, data are limited for patients that are currently taking low or no doses of ACEI or ARB. Therefore, current recommendations for these patients are to start with a dose of 50mg twice daily and to titrate the dose slowly (doubling every 3-4 weeks).
Third, sacubitril/valsartan should not be coadministered with an ACEI or an ARB. When used concomitantly with an ACEI, there is a high potential risk of angioedema. Consequently, sacubitril/valsartan must not be started for at least 36hours after discontinuing ACEI therapy.
Fourth, no dose adjustment is required in patients with mild renal impairment (estimated glomerular filtration rate 60-90mL/min/1.73 m2). However, a starting dose of 50mg twice daily should be considered in patients with moderate renal impairment (estimated glomerular filtration rate 30-60mL/min/1.73 m2). There are no data on patients with end-stage renal disease, but the use of sacubitril/valsartan is not recommended for these patients.
Fifth, caution should be exercised when initiating sacubitril/valsartan in patients with NYHA functional classification IV, due to the limited clinical experience in this population.
Sixth, BNP is not a suitable biomarker of heart failure in patients treated with sacubitril/valsartan, because it is a neprilysin substrate. Switching to NT-proBNP as a natriuretic peptide biomarker is recommended.
Seventh, a theoretical risk associated with neprilysin inhibition is related to the accumulation of the neprilysin substrate, amyloid-β, in the brain.8 No increased incidence of cognition- or dementia-related adverse events were reported in the PARADIGM-HF trial. However, these effects may not have been detected to date, because dementia may take longer to develop than the current period of observation of participants in the trial. Also, subjects with mild dementia were not expected to participate. However, the ongoing Phase III PARAGON-HF trial has implemented a Cognitive Function Assessment.
Eighth, coadministration of sacubitril/valsartan and atorvastatin increased the Cmax of atorvastatin and its metabolites by up to 2-fold. There were no significant increases in potential statin-related adverse events in the patients who received both sacubitril/valsartan and statin in the PARADIGM-HF trial. Nevertheless, further analyses have shown that higher doses of statins were associated with more adverse events, when combined with either sacubitril/valsartan or enalapril. However, the patterns were different, depending on the specific statin administered. Pending the results of further studies, caution has been recommended for this drug combination.7
The PARADIGM-HF trial is focused on chronic heart failure with limited LVEF. Thus, the question arises: What about the other 50% of patients with heart failure, but preserved ejection fraction, also known as HFpEF patients? Currently, there is a lack of clinical trials on HFpEF that demonstrated therapeutic benefits with agents commonly used in patients with reduced ejection fraction. Consequently, therapies for HFpEF are directed toward symptom management and cardiovascular risk factors. However, among patients with HFpEF, sacubitril/valsartan showed promising safety and efficacy results in a phase 2 trial. The PARAMOUNT trial was a randomized, double-blind, parallel-group, active controlled trial that compared sacubitril/valsartan with valsartan alone.9 The primary endpoint was a change from baseline in NT-proBNP at 12 weeks. The groups had similar baseline characteristics. Most patients were aged, female, overweight, and classified as NYHA class II. A greater NT-proBNP reduction was detected at week 4 in the sacubitril/valsartan group compared with the valsartan group, but it did not reach significance (P = .063). At 12 weeks, NT-proBNP was significantly reduced in the sacubitril/valsartan group compared with valsartan (P = .005). The findings from PARAMOUNT suggested that sacubitril/valsartan might have favorable effects in patients with HFpEF. Further investigation of the HFpEF population is ongoing in the PARAGON study, a multicenter, randomized, double-blind, parallel group, active controlled study. That study aims to evaluate the efficacy and safety of sacubitril/valsartan compared with valsartan on morbidity and mortality in patients with heart failure (NYHA class II-IV) and preserved ejection fraction.
Last, but not least, very recently, circulating soluble neprilysin (sNEP) was proposed as a putative biomarker.2 At present, data on sNEP have suggested that it may play a prognostic role in both chronic10 and acutely decompensated heart failure,11 but in HFpEF results are controversial.12 Interestingly, circulating sNEP was shown to be catalytically active.13 Moreover, a recent report demonstrated that sNEP might even be superior to NT-proBNP as a surrogate prognostic biomarker of the neurohormonal axis in heart failure.14 Further refinements in sNEP assays are mandatory before its introduction to clinical practice. However, the data reported to date suggest that it may become a valuable tool for patient prognostication and eventually therapy guidance.
As an epilogue, the cost of the treatment with this new agent is likely to represent a barrier to its use in everyday real-life clinical practice, since the cost of effective agents such as the ACEI enalapril is very low (comparable to the cost of chewing gum in many countries). Conceivably, the implementation of a biomarker-driven strategy may be proposed to preferentially switch from ACEI treatment to Entresto in the sickest patients. Along these lines, it is noteworthy that the use of natriuretic peptides was among the inclusion criteria in the PARADIGM-HF trial. The cost-effectiveness and cost per quality-adjusted life year gained of sacubitril/valsartan relative to enalapril for treatment of HFrEF deserve intensive research in real-world scenarios adjusted by country and health care system.15
CONFLICTS OF INTERESTA. Bayes-Genis and J. Lupón have applied for a patent for sNEP as a prognostic biomarker, which is pending approval. A. Bayes-Genis has lectured and participated in Advisory Boards from Novartis.