The new guidelines on heart failure (HF) presented by the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) at the latest ESC congress were possibly the most eagerly awaited guidelines in the history of HF because they had to consider the major advances made in the last 5 years, particularly regarding HF with reduced ejection fraction (HFrEF).1 These advances have reduced HF hospitalizations and improved the prognosis and quality of life of patients with HF.
The clinical evidence on neprilysin inhibitors combined with conventional neurohormonal therapy (angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin II receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists [MRAs] and the subsequent implementation of sodium-glucose cotransporter 2 [SGLT2] inhibitors) has guided clinical practice toward 5 therapeutic targets (sympathetic nervous system, renin-angiotensin system, aldosterone, neprilysin, and metabolic/myocardial performance). This global therapeutic approach was achieved with what has been named foundational therapy: angiotensin receptor-neprilysin inhibitors (ARNIs), beta-blockers, MRAs, and SGLT2 inhibitors.2
These new guidelines also note the impact of the COVID-19 pandemic on various trials, which failed to achieve their targets due to limitations concerning patient recruitment and follow-up logistics.
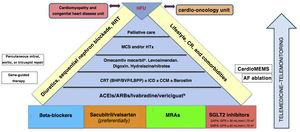
Table 1 shows the most important novelties while figure 1 illustrates their integration into clinical practice.
Notable novelties in the new HFA-ESC 2021 guidelines on heart failure
| • Change of the HFmEF category to that of HFmrEF |
| • A simplified treatment algorithm for HFrEF with drugs and devices that have a class I recommendation |
| • 4 key drug treatments: ACEIs or ARNIs, beta-blockers, MRAs, and SGLT2 inhibitors should be started as soon as it is possible and safe |
| • The SGLT2 inhibitors dapagliflozin and empagliflozin have a class I recommendation |
| • Grouped and stepwise treatment algorithm for HFrEF by phenotype for individualized management |
| • Vericiguat, a guanylate cyclase stimulator, is a class IIb recommendation |
| • Primary prevention with ICD implantation in nonischemic cardiomyopathy is a class IIa recommendation |
| • Emphasis on the degree of the QRS width in LBBB for the selection of patients for CRT |
| • Inclusion of a recommendation table for the management of HFmrEF |
| • Regarding the management of HFpEF, the recommendations of the new guidelines have been maintained largely without changes but require reassessment given the subsequently published therapeutic novelties |
| • Modified classification of the forms of presentation of AHF |
| • Adjusted assessment of the congestion state and OMT optimization for HF before hospital discharge |
| • Plan with an early visit an adequate transition to discharge after HF hospitalization |
| • Improved level of recommendation for long-term MCS as bridge to transplant or destination therapy in advanced HF |
| • For the first time, heart transplantation receives a class I recommendation with level of evidence C |
| • Updating of the treatments for noncardiovascular comorbidities: diabetes mellitus, iron deficiency, hyperkalemia, and cancer |
| • Updating of the recommendations for percutaneous or surgical treatments in mitral and aortic valve diseases |
| • Tafamidis is a class I recommendation in patients in New York Heart Association classes I and II and with transthyretin cardiac amyloidosis |
| • Indication for genetic testing in the evaluation of cardiomyopathies |
| • Recommendation for the self-care and follow-up/monitoring of patients with HF via home- or hospital-based programs |
| • Inclusion in the guidelines of quality indicators for HF |
ACEIs, angiotensin-converting enzyme inhibitors; AHF, acute heart failure; ARNIs, angiotensin receptor-neprilysin inhibitors; CRT, cardiac resynchronization therapy; HF, heart failure; HFA-ESC, Heart Failure Association of the European Society of Cardiology; HFmEF, heart failure with mid-range ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; ICD, implantable cardioverter-defibrillator; LBBB, left bundle branch block; MCS, mechanical circulatory support; MRAs, mineralocorticoid receptor antagonists; OMT, optimal medical therapy; SGLT2, sodium-glucose cotransporter-2.
Integrated approach to patients with heart failure with reduced ejection fraction (HFrEF). ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; BHP, bundle of His pacing; BVP, biventricular pacing; CCM, cardiac contractility modulation; CR, cardiac rehabilitation; CRT, cardiac resynchronization therapy; DAPA, dapagliflozin; EMPA, empagliflozin; HFU, heart failure unit; HTx, heart transplantation; GFR, glomerular filtration rate; ICD, implantable cardioverter-defibrillator; ID, iron deficiency; LBB, left branch block; LBBP, left bundle branch pacing; MCS, mechanical circulatory support; MRAs, mineralocorticoid receptor antagonists; RRT, renal replacement therapy; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter-2. aGALACTIC (NCT02929329): omecamtiv mecarbil if SBP ≥ 85mmHg or GFR ≥ 20mL/min/1.73 m2 (not licensed). bVICTORIA (NCT02861534): vericiguat if SBP ≥ 100mmHg or GFR ≥ 15mL/min/1.73 m2.
The updated guidelines and the publication of the new universal definition of HF1,3 maintain 3 categories based on the phenotypes of patients with different prognoses and treatments. Two of these categories are already quite consolidated, namely, those of HFrEF if left ventricular ejection fraction (LVEF) is ≤ 40% and HF with preserved ejection fraction (HFpEF) when LVEF is ≥ 50%; the third category involves a new nomenclature for patients who were previously classified as having “midrange” LVEF values (between 41% and 49%) and who are now considered to have HF with mildly reduced LVEF (HFmrEF). This change means that these patients with HFmrEF receive very similar treatment to that of patients with HFrEF, although the level of recommendation for all drugs is class IIb and level of evidence C.1
Identification of the HF etiology is required to determine the specific therapeutic approach. In these guidelines, the recommendation for invasive coronary angiography has been relegated to IIb. This procedure should be considered in patients with HFrEF who have a moderate-to-high pretest probability of coronary heart disease (CHD) and stroke in a noninvasive stress test1; this recommendation was class IIa in the 2016 guidelines.4 In addition, coronary computed tomography angiography is assigned a class IIa recommendation in patients with a moderate-to-low pretest probability of CHD or in those with inconclusive noninvasive stress test results that conclusively rule out CHD; this recommendation was class IIb in the 2016 guidelines.4
Moreover, right heart catheterization should be considered in patients with HF and diagnostic suspicion of constrictive pericarditis, congenital heart disease, and high output states (class IIa). Right heart catheterization is also recommended (class IIb) in selected patients with HFpEF to confirm the diagnosis.1
NEW DRUG THERAPY RECOMMENDATIONSHeart failure with reduced ejection fractionThe new guidelines propose a stepwise treatment algorithm for patients with HFrEF based on quadruple therapy (combination of ACEIs/ARNIs, beta-blockers, MRAs, and SGLT2 inhibitors) as the first step in treatment and with a maximum level of recommendation (I A) for all drugs, except for ARNIs (I B).1 Implementation of this foundational therapy remains an art and should not necessarily be conducted in a single day, but its early implementation must be an objective (ideally within 4 weeks) to reach the doses described in the literature. This strategy requires individualization and identification of the different phenotypes of patients, as has also been published in the American consensus document5 and in the Canadian guidelines.6
The clinical impact of the foundational therapy is based on the excellent outcomes with SGLT2 inhibitors (dapagliflozin and empagliflozin) in the DAPA-HF7 and EMPEROR-Reduced8 trials, which included patients with HFrEF and showed a significant reduction in cardiovascular mortality and/or HF hospitalizations. The evidence on SGLT2 inhibitors has been strengthened by the results on acute HF in the SOLOIST-WHF trial.9
The present guidelines assign a class IIb recommendation to vericiguat, a guanylate cyclase stimulator that increases the concentration of cyclic guanosine monophosphate. In the VICTORIA trial,10 vericiguat was effective in patients with HF worsening and previous hospitalization events despite optimal medical therapy (OMT), with a 10% fall in the composite endpoint of cardiovascular mortality and first hospitalization for HF, mainly due to a greater impact on hospitalizations.
The guidelines do not make a recommendation on omecamtiv mecarbil, a selective cardiac myosin activator, due to doubts about its subsequent licensing. In the GALACTIC-HF trial,11 with more than 8000 patients with HFrEF and clinical severity criteria, omecamtiv mecarbil showed a slight reduction of 8% in the composite end point of cardiovascular death, HF hospitalizations, and emergency department visits. In subgroup analysis, patients with more severe symptoms and worse LVEF appeared to benefit most from omecamtiv mecarbil.11
Heart failure with preserved ejection fractionThese guidelines,1 as in those of 2016,4 recommend the screening and management of the causes and the cardiovascular and noncardiovascular comorbidities of patients with HFpEF. Regarding drug therapy, a class I recommendation with level of evidence C is maintained for diuretic therapy to improve congestive signs and symptoms. Unfortunately, the authors could not include the impactful outcomes of the EMPEROR-Preserved12 trial with empagliflozin, the first positive study of patients with HFpEF; one-third of the patients included in this study had HFmrEF. The results of the EMPEROR-Preserved trial have once again focused the controversy on the different LVEF cutoff points at the time of patient classification, and definition of a “normal” LVEF is required.
Regarding the diagnostic criteria of HFpEF, the guidelines stress the adoption of the positioning proposals of the ESC, whose most important novelties lie in the performance of diastolic exercise stress testing together with the reference standard for diagnosis: exercise right heart catheterization.1
Acute heart failureThere is a new classification of acute HF based on 4 forms of presentation (decompensated HF, pulmonary edema, cardiogenic shock [CS], and isolated right ventricular failure), each requiring specific management. In the initial phase of treatment, intensive management with intravenous loop diuretics is recommended, guided by diuresis and the urine concentration of sodium, before the use of diuretic combinations, with the recommendation changing from IIb to IIa. For patients with CS, noradrenaline is better than adrenaline and, given the neutral results of new studies with vasodilators in patients with acute HF, the recommendation has dropped from IIa to IIb. Finally, before hospital discharge, an adjustment of the OMT is required, as well as the titration and planning of an appropriate transition to discharge with an early visit, in the first week after discharge if possible.1
Advanced heart failure, mechanical circulatory support, and heart transplantationThis section has had a major impact on the new guidelines, particularly on the importance of the generation of clinical pathways and treatment algorithms for patients with advanced HF criteria in order to refer them to specialized centers for the evaluation of treatments such as mechanical circulatory support (MCS) and heart transplant. In the section on short-term MCS, the recommendation is upgraded from IIb to IIa in patients with CS as bridge to decision or to recovery or in heart transplant candidates.1 An important point is the need to organize care pathways for patients with CS via the creation of referral centers equipped with multidisciplinary teams.1
In the case of long-term MCS, the patients considered should have a history of treatment adherence, appropriate capacity for device handling, and psychological support. The recommendation has been upgraded for long-term MCS as bridge to heart transplant and as destination therapy due to the excellent outcomes of the MOMENTUM 3 trial13 with the HeartMate3 (Abbott Labs, United States), a pump that exhibits improved hemocompatibility. The greater than 80% 2-year survival in that study has led to a class IIa recommendation in the guidelines.1
It is important to note that, for the first time in the history of the HF guidelines, heart transplant is a class I recommendation with level of evidence C.1
NONDRUG THERAPY RECOMMENDATIONSImplantable cardioverter-defibrillators and cardiac resynchronization therapyOne of the most important changes in the 2021 guidelines1 is the lower level of recommendation for implantable cardioverter-defibrillator implantation in patients with nonischemic cardiomyopathy, which is relegated from I to IIa; it remains class I for ischemic patients.
In addition, if the QRS is between 130 and 149ms and in the presence of left bundle branch block with LVEF < 35% and OMT, the indication for cardiac resynchronization therapy has decreased from I to IIa, while that with QRS > 150ms is class I, which underlies the importance of QRS widening at the time of the clinical indication for cardiac resynchronization therapy.1 An aspect requiring further exploration in the next guidelines is the role of physiological pacing and its potential indications beyond patients with cardiac resynchronization therapy failure with the standard technique.
COMORBIDITIESThe novelties in the guidelines regarding comorbidities are summarized in this section and are particularly focused on:
- 1.
Iron deficiency. The novelty lies in the recommendation (IIa) for ferric carboxymaltose to reduce HF hospitalizations, based on the AFFIRM-AHF study.14 In that study, intravenous ferric carboxymaltose in patients with iron deficiency and decompensated HF with LVEF < 50% was associated with a significant reduction in HF hospitalizations with no improvement in mortality.14
- 2.
Type 2 diabetes mellitus. SGLT2 inhibitors have a class effect for preventing HF hospitalizations and cardiovascular mortality and reducing cardiovascular events and progression to advanced kidney disease. In addition, all patients with HF and type 2 diabetes mellitus should be treated with an SGLT2 inhibitor (dapagliflozin, empagliflozin, sotagliflozin), a class I A recommendation.1
- 3.
Atrial fibrillation (AF). Direct oral anticoagulants have a higher recommendation (I vs IIa) than warfarin in patients with AF, HF, and a CHA2DS2-VASc score ≥ 2 (men) or ≥ 3 (women). Catheter ablation of AF has an upgraded recommendation (from IIb to IIa) in patients exhibiting a clear relationship between HF worsening and persistent or paroxysmal AF despite OMT.1
- 4.
Surgical coronary revascularization. This is the procedure of choice in patients with concomitant type 2 diabetes mellitus, HFrEF, and multivessel disease (IIa B). In addition, coronary revascularization surgery should be considered for symptom improvement in patients with persistent angina despite OMT with antianginal agents in patients with LVEF < 40%, CHD, and anatomy suitable for revascularization, a class IIa recommendation (previously class I).1
- 5.
Severe aortic stenosis. The indication for transcatheter aortic valve implantation or surgical aortic valve replacement in patients with a severe transaortic gradient receives the maximum recommendation (I B) but the choice between the techniques depends on the heart team assessment and patient's preferences (I C).1
- 6.
Severe mitral regurgitation. Percutaneous edge-to-edge mitral valve repair via MitraClip implantation (Abbott Vascular, United States) should be considered in selected patients with secondary mitral regurgitation not eligible for surgery but with no need for coronary revascularization who are symptomatic despite OMT and also meet criteria for a reduction in hospitalizations (IIa B).1
- 7.
Cancer. Patients with cancer and increased risk of cardiotoxicity should be evaluated in cardio-oncology units (I C).1
The most novel aspects are the value of genetic studies in the diagnostic and prognostic assessment of patients with cardiomyopathy.1 In addition, there is a class I recommendation for tafamidis, which was evaluated in the ATTR-ACT trial,15 for cardiac amyloidosis and wild-type and hereditary transthyretin-related cardiomyopathy in the context of New York Heart Association class I or II symptoms to reduce symptoms, cardiovascular hospitalization, and death.1
HEALTH CARE MODELS AND TRAININGThe importance of organization in HF programs is particularly evident in these guidelines1: cardiac rehabilitation is part of the treatment algorithm to reduce hospitalizations and improve quality of life (I A)1; home-based HF care programs or those in specialized HF clinics improve the course and reduce the risk of HF hospitalizations and death (I A)1; and patient training in self-care (I A) together with the application of telemedicine and telemonitoring are also key to reducing morbidity and mortality. Also vital is the recommendation to vaccinate our patients against the influenza virus and pneumococcus (IIa B).1 Unfortunately, these guidelines do not refer to the randomized and multicenter Spanish study ETIFIC,16 which showed the noninferiority of nursing staff specialized in HF in the titration and optimization of HF treatment vs cardiologists specialized in HF.
FUTURE OF CLINICAL PRACTICE GUIDELINESFinally, we must reflect on the clinical practice guidelines of the future in a digital world with almost instantaneous access to scientific information.17 The publication of these guidelines before the presentation of one of the studies12 expected to modify the clinical practice in patients with HFpEF challenges their validity in this field and gives the impression of guidelines that are already obsolete at the time of their publication. Accordingly, it is time to imagine new proposals for the future, without forgetting that guidelines have been and will be one of the major advances of modern medicine and that they are the basis of evidence-based medicine.
FUNDINGThis editorial has not received any funding.
CONFLICTS OF INTERESTN. Manito has performed consultancy work and conferences for Novartis, AstraZeneca, Boehringer Ingelheim, Bayer, Vifor Pharma, and Abbott.
I thank CERCA Programme/ Generalitat de Catalunya for institutional support.