Keywords
INTRODUCTION
Cardiovascular disease is a major cause of morbidity and mortality in the western hemisphere.1 Despite major advances in the management and diagnosis of patients with coronary artery disease, a large number of victims who are apparently healthy die suddenly without prior symptoms.2,3 Most of these events are related to plaque rupture and subsequent thrombotic occlusion at the site of non-flow limiting atherosclerotic lesions in epicardial coronary arteries.4,5 In addition, silent plaque rupture and its subsequent wound healing accelerate plaque growth and are a more frequent feature in arteries with less severe luminal narrowing.6
According to histological studies, plaque composition plays a central role in the pathogenesis of epicardial occlusion, irrespective of the severity of the underlying stenosis.5
THE IMAGING TARGET: THE THIN-CAP FIBROATHEROMA
Recently, retrospective studies have identified morphological and compositional characteristics of plaques prone to rupture.7,8 This has lead to a new classification of coronary lesions that depicts plaque progression in a more comprehensive manner.8
Thin-cap fibro atheroma (TCFA) lesions, the most prevalent predecessor of plaque rupture, are composed of a lipid-rich atheromatous core, a thin (≤65 μm) fibrous cap with macrophage and lymphocyte infiltration, decreased smooth muscle cell content and expansive remodeling.8,9
Detection of these non-obstructive, lipid rich, high-risk plaques may have an important impact on the prevention of acute myocardial infarction and sudden death.
Although angiography can identify obstructive as well as complex lesions,10 it is restricted to the visualization of the coronary lumen and is unable to visualize the coronary wall. Thus, features as vessel remodeling or plaque composition are missed. Recently, a post-mortem study evaluated the geometrical aspect of the vessel wall and showed a relationship between local alterations of vessel size and plaque stability.11
Currently, there are several intravascular tools capable of locally evaluating determinants of plaque vulnerability such as the size of the lipid core, thickness of the fibrous cap, inflammation within the cap and positive remodelling.
A recent study proposed a critical cap thickness of <65 micron based on post mortem histomorphometry.12 However, in vivo the threshold for defining a fibrous cap as thin should probably be higher than 65 μm for several reasons. First, it is well established that general tissue shrinkage can not be avoided during histologic fixation which implies dehydration processes.13,14 Furthermore, circumferential stress at the luminal border of the plaque increases critically when cap thickness is less than approximately 150 μm.15
We summarize the current status of imaging techniques that have the potential to detect the vulnerable plaque features in vivo and may allow risk stratification in a specific individual and ultimately guide systemic and local preventive strategies.9,16-20
INTRAVASCULAR ULTRASOUND
Gray scale IntraVascular UltraSound (IVUS) is an invasive diagnostic tool that provides a real-time, high-resolution, tomographic view of coronary arteries. It thereby enables the assessment of morphology, severity and extension of coronary plaque.
IVUS is currently the only imaging modality that can provide in vivo information regarding temporal changes in the atherosclerotic plaque size.21
Qualitative plaque characterization is based on the echogenicity of the received ultrasound signal, whereas echolucent zones reflect lipid-rich tissue and highly reflective structures with dorsal shadowing calcified tissue. Nevertheless, plaque characterization through visual interpretation of gray-scale IVUS is imprecise, specially when assessing heterogeneous, lipid-rich plaques.22
Axial resolution is limited to 100-200 μm thus impairing the ability of detecting thin fibrous caps. Notwithstanding, for the aforementioned reasons, we believe that the threshold for defining a fibrous cap as thin should be higher than 65 μm.
The detection of vulnerable plaques by IVUS is mainly based on a series of case reports.23-26 These reports describe morphologic features of already ruptured plaques but not the prospective detection of rupture-prone plaques. Nevertheless, one prospective study showed that large eccentric plaques containing an echolucent zone by IVUS were found to be at increased risk of instability even though the lumen area was preserved at the time of initial study.27
ROLE OF VESSEL REMODELING
Vascular remodeling was described by Glagov as a compensatory enlargement of the coronary arteries in response to an increase in plaque area.28 Several studies showed an increase level of inflammatory marker levels, larger lipid cores and pronounced medial thinning in positive remodeled vessels.11,29,30 This concept has further evolved to a dynamic theory where vessels may also shrink in response to plaque growth.31 This remodeling modality has been related to a more stable phenotype and clinical presentation.11,29,32,33 Recently, the relationship between vascular remodeling and plaque composition was assessed using IVUS.34-36 In these studies, the remodeling index for soft lesions was significantly higher than those for fibrous/mixed and calcified lesions.34-36 It is noteworthy though, that most studies evaluating this phenomenon are of cross sectional design. Since atherosclerosis is usually a diffuse disease, finding a fully non-diseased reference is not guaranteed. Therefore, the early presence of remodeling in the reference site can't be ruled out.
QUANTITATIVE IVUS ECHOGENICITY ASSESSMENT
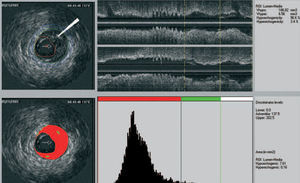
We recently developed a computer-aided, gray-scale value, analysis program for plaque characterization.37 Based on the mean gray level (brightness) of the adventitia, plaque is classified as more (hyperechogenic) or less bright (hypoechogenic) in relation to the adventitia (Figure 1). The percentage of hypoechogenic plaque is calculated for the entire region of interest and for slices with significant plaque. In the carotid circulation, plaque echogenicity, measured noninvasively, has been related to the histological components of plaque.38-41 Furthermore, carotid plaque echolucency (low echogenicity) was associated with future neurological events.42-44 IVUS-based plaque characterization in the coronary circulation requires invasive assessment and has been less extensively studied. A recent study showed that treatment with atorvastatin resulted in quantifiable changes in coronary plaque echogenicity, compatible with changes in plaque composition.45 These findings offered a potential explanation for the clinical efficacy of statins despite only modest effects on plaque volume.21,46 Both ex vivo and clinical studies that will provide validation data about the technique are currently in progress.
Figure 1. IVUS echogenecity: the adventitia is defined as tissue outside the external elastic membrane. For all non-shadowed adventitia pixels, the mean value and standard deviation are calculated. To observe the suitability, a normal distribution curve based on the same mean and standard deviation histogram is created. Hypoechogenic areas are colored red and hyperechogenic areas green.
Intravascular Ultrasound Elastography and Palpography
An important patho-morphologic feature of vulnerable plaque is
the eccentric accumulation of a lipid-rich necrotic core within the vessel wall, separated from the lumen by a thin fibrous cap. This observation led to the hypothesis that vulnerable lesions might have mechanical properties that differ from those of chronic stable lesions. Intravascular ultrasound elastography and palpography are techniques that allow the assessment of local mechanical tissue properties.19,47
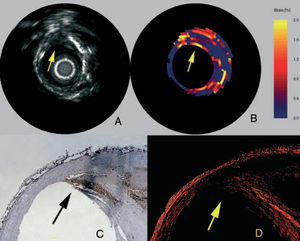
At a defined pressure, soft tissue (lipid-rich) components will deform more than hard tissue components (fibrous-calcified).48 In coronaries, the tissue of interest is the vessel wall, whereas the blood pressure with its physiologic, systolic and diastolic changes during the heart cycle is used as the excitation force. Images obtained at different pressure levels are compared to determine the local tissue compression. The radial strain in the tissue is calculated by cross-correlation techniques on the radio frequency signal and can be displayed as a colour-coded image.48 The sensitivity and specificity to detect vulnerable plaques has recently been assessed in post-mortem human coronary arteries where vulnerable plaques were detected with a sensitivity of 88% and a specificity of 89% (Figure 2).19 In addition to ex-vivo studies, this technique has also been tested in vivo, where palpography detected a high incidence of deformable plaques in acute coronary syndrome (ACS) patients. Furthermore, the number of highly deformable lesions was correlated to the clinical presentation and levels of C-reactive protein.47
Figure 2. Vulnerable plaque marked in IVUS (A), elastogram (B), macrophage staining (C), and collagen staining (D). In the elastogram, a vulnerable plaque is indicated by a high strain on the surface. In the corresponding histology, a high amount of macrophages (C) is visible with a thin cap (D) and a lipid pool (LP). (Schaar et al. Circulation. 2003;108:2636.)
The main limitation of the technique is that it depends on the quality and stability of the coronary pressure signal. Accordingly, it might be disturbed by high heart rates and rhythm disturbances.
Virtual Histology
Gray-scale IVUS is of limited value for identification of specific plaque components.49 Calcified and dense fibrous tissues usually are highly echo-reflective thus calcified areas are commonly overestimated. On the other hand, low echo-reflectance plaques are considered "soft" or lipid-rich. However, the accuracy of gray-scale IVUS for discriminating lipid from fibrous tissue is limited since in addition to large amounts of extracellular lipids (low echo-reflective areas), the lipid core contains cholesterol crystals, necrotic debris and microcalcifications (highly echoreflective areas).8
A recently introduced technique (IVUS-Virtual HistologyTM [IVUS-VH], Volcano Therapeutics, Rancho Cordova, CA) that uses the substrate (frequency domain analysis) of the IVUS radiofrequency (RF) data rather than the envelope (amplitude), has demonstrated its potential to provide an objective and accurate assessment of coronary plaque composition in studies of explanted human coronary segments.20
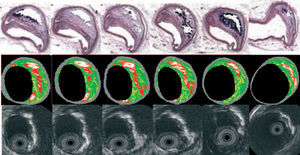
IVUS-VH uses spectral analysis of IVUS radiofrequency data to construct tissue maps that classify plaque into four major components. In preliminary in vitro studies, four histological plaque components (fibrous, fibrolipidic, lipid core, and calcium) were correlated with a specific spectrum of the radiofrequency signal.20,50 These different plaque components were assigned color codes. Calcified, fibrous, fibrolipidic, and lipid core regions were labeled white, green, greenish-yellow, and red respectively (Figure 3).
Figure 3. Serial histological sectioning of a coronary vessel. The middle and below panels depict the cross-correlation with Virtual HistologyTM and gray-scale IVUS respectively. Calcified, fibrous, fibrolipidic and lipid core regions are labeled white, green, greenish-yellow and red respectively.
IVUS studies have failed to conclusively demonstrate regression in plaque burden over time.21,51,52 IVUS-VH has, though, the potential to follow the progression of the disease not only with regards to its volume, but to its composition as well.53 Moreover, this tool could also be helpful in evaluating the effect of both conventional and emerging therapeutic interventions.
With regards to vulnerable plaque detection, IVUS-VH allows an accurate and quantitative assessment of 2 of the main features of the TCFA: lipid core and positive remodeling.
A main limitation of this technique is its inability to detect thin fibrous caps. However, as aforementioned we believe that the threshold for defining a thin fibrous cap should be higher than 65 μm.
Optical Coherence Tomography
Optical coherence tomography (OCT) is an imaging technique that allows high-resolution (axial resolution of 15 μm) imaging in biological systems.54 Accordingly, OCT has the capacity to allow in vivo, real time visualization of a thin fibrous cap. OCT imaging is based on low coherence near infrared light that is emitted by a superluminescent diode. A center wave length around 1300 nm is used since it minimizes the energy absorption in the light beam caused by protein, water, haemoglobin, and lipids. The light waves are reflected by the internal microstructures within biological tissues as a result of their differing optical indices.
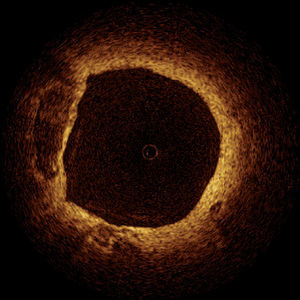
Animal and Post-mortem studies demonstrated the accuracy of OCT in comparison to histology.55-57 These studies showed that OCT can detect both normal and pathologic artery structures (Figure 4).57 Recent in vivo data have shown that OCT can differentiate different plaque types and suggested the possibility of detection of macrophages in atherosclerotic plaques.58,59
Figure 4. Optical coherence tomography of a non-flow limiting lesion showing a "swiss-cheese" vessel wall suggestive of a thin-cap fibroatheroma.
In our experience, TCFA with low-reflecting necrotic cores covered by highly reflecting thin (mean 50 μm) fibrous caps cap can be visualized in patients scheduled for percutaneous coronary intervention (PCI).60
The high resolution of OCT offers the potential to detect TCFA in living patients.59,60 OCT imaging, however, is limited by the relative shallow penetration depth that hampers imaging of the entire vessel wall in medium and large vessels large vessels, and the need to clear the artery from blood during imaging causing transient ischemia of the studied region.
Intravascular Thermography
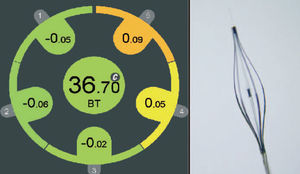
The rationale to measure vascular temperature is based on the observation that atherosclerosis is accompanied by inflammation. Vulnerable plaques have been associated with increased macrophage activity, metabolism and inflammation.61 Based on these findings the hypothesis was generated that these "activated" macrophages produce thermal energy, which might be detected on the surface of these atherosclerotic lesions. Infrared and contact-sensor thermographies are the most important invasive methods (Figure 5). The contact thermographic methods seem to be the most feasible at the present time, mainly due to the difficulties of infrared radiation to penetrate the flowing blood to detect vessel wall temperature. A small study of 19 patients that included patients with stable angina, unstable angina, and with acute myocardial infarction reported temperature heterogeneity in human atherosclerotic coronary plaques.62 Intracoronary temperature was assessed using a dedicated catheter. In most coronary segments with atherosclerotic plaques a rise in temperature was seen as compared to coronary segments with a normal vessel wall. Temperature differences between an atherosclerotic plaque and a normal vessel wall increased progressively from patients with stable angina to patients with acute myocardial infarction with a maximum temperature difference to the background temperature of 1.5±0.7°C. However, there are somewhat conflicting published and unpublished reports with other thermography devices (circular basket or self expanding arms) that have documented a much lower heterogeneity of temperature distribution. The most likely explanation for this discrepancy in temperature observations might be related to the difference in catheter design and the way coronary flow is affected.
Figure 5. Dedicated thermography catheter with 5 thermistors in contact to the vessel wall showing significant heterogeneity in the measurements compatible with increased macrophage activity, metabolism, and inflammation.
These preliminary findings about the thermal status of atherosclerotic plaques seem promising. However, accurate temperature evaluation requires direct contact of the thermistors with the vessel wall, carrying the potential risk of endothelial damage. In addition, the cooling effect caused by blood flow may hamper data interpretation.63
Angioscopy
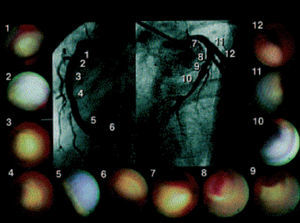
This technique allows real-time direct visualization of coronary plaques (Figure 6). Ex vivo validation of angioscopy was performed by Thieme et al, who compared angioscopic observations with histologic samples obtained by coronary atherectomy. In this study, yellow plaques were related to atheromatous lesions.64 These findings were confirmed in clinical studies, where lipid-rich, rupture-prone plaques were easily detected by angioscopy as yellow plaques, and found more commonly in acute coronary syndromes.65 Furthermore, angioscopy has shown intriguing results in the prediction of acute coronary syndromes.65
Figure 6. Patient with anterior myocardial infarction. Angioscopic images of the culprit lesion (8 and 9) and of all the yellow plaques in the non-culprit segments are presented. Thrombus was detected over the yellow plaque in the culprit segment. (Asakura et al. J Am Coll Cardiol. 2001;37:1284-8.)
Despite these encouraging findings, this technique examines solely the luminal surface of the intima. Thus, key TCFA features such as thickness of the cap, lipid core content, and remodelling can not be assessed. In addition, blood must be cleared away from the view causing transient ischemia of the studied region.
RAMAN SPECTROSCOPY
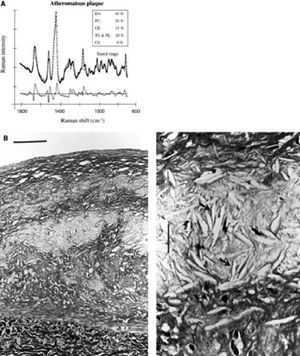
Raman spectroscopy is a technique that can characterize the chemical composition by means of the Raman effect.66 This effect is created when incident light excites molecules in a tissue sample, which scatter light in a different wave length. This change in wave length called the "Raman effect" is dependent on the chemical components of the tissue sample. Thus, Raman spectroscopy can provide quantitative information about molecular composition of a sample.67 The spectra obtained require post-processing to differentiate between plaque components (Figure 7). In vitro studies have demonstrated that diagnostic algorithms allow the discrimination of coronary arterial tissue in 3 categories: non-atherosclerotic, non-calcified and calcified plaques.67
Figure 7. Raman spectrum from atheromatous plaque and model fit (A). The increase of the relative weights of the chemical components FC and CE, as compared with intimal fibroplasia, corresponds to the presence of an atheromatous core under a fibrous cap (B, bar indicates 100 μm). An abundance of lipid-laden foam cells (open arrows) and FC crystal clefts (solid arrows) is visible in the atheromatous core (C, bar indicates 25 μm). (Romer et al. Circulation. 1998;97:878-85.)
The main limitations of the technique are the inability to provide geometrical information, the narrow penetration depth (1.0 to 1.5 mm) and the absorbance of the laser light by the blood.
INTRAVASCULAR MAGNETIC RESONANCE IMAGING
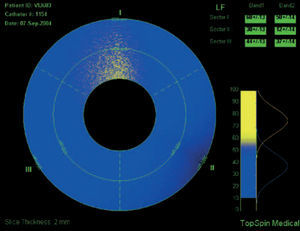
Intravascular magnetic resonance imaging (MRI) is another potential approach to determine plaque composition based on the diffusion properties of the analyzed tissue. MRI can determine the presence of lipid within the arterial wall. Current technology for intravascular MRI consists of a self-contained MRI probe that allows sequential scanning of different vessels sectors. The lipid-content within a sector is determined and the data is displayed color-coded (yellow corresponds to high lipid content within the region of interest, blue to low lipid-content [Figure 8]).
Figure 8. Intravascular MRI of a coronary vessel where plaque composition is colo-coded: yellow corresponds to high lipid content within the region of interest, blue to low lipid (fibrotic) content.
The intravascular MRI system has been evaluated in ex-vivo human carotid tissue, aortic tissue and coronary arteries to correlate MRI findings with histology. In ex vivo aortic studies the MRI correctly predicted the histologic results in 15 of 16 aortic cases, and in ex-vivo coronary arteries 16 of 18 lesions were correctly predicted, including the diagnosis of 3 thin cap fibroatheromas.68,69 In vivo feasibility is currently under investigation in a multi-center trial.
FUTURE PERSPECTIVES
It has previously been shown that a multifocal instability process is present in acute coronary syndromes.26,70 Rioufol et al demonstrated in ACS patients that at least one plaque rupture is found away from the culprit lesion in 80% of the patients, away from the culprit artery in 71% and in the 2 non-culprit arteries in 12.5%.26
The large number of high-risk lesions found throughout the coronary tree by means of angiography,71 angioscopy,70 IVUS,26 and palpography47 in addition to the unpredictability of the natural history of such lesions and the uncertainty about if vulnerable plaque characteristics might subsequently lead to fatal or non-fatal ischemic events, suggests that potential local preventive strategies could not be cost-effective.
However, high-risk "yellow" plaques identified in stable patients by angioscopy have been found predictors of ischemic events.65 Accordingly, a systemic approach including intensive statin therapy could be a reasonable approach to "cool-down" the inflammatory burden.
Although enormously promising, catheter-based techniques need more extensive validation and an appropriate vulnerable plaque model is yet to be developed. In addition, these techniques interrogate the coronary arteries in a localized manner, whereas inflammation is distributed throughout the whole coronary tree.72
These new modalities have the potential to provide insights into pathophysiology in studies of the natural history of coronary plaque. Furthermore, they may provide surrogate endpoints. Finally, the combination between novel imaging techniques and the assessment of circulating biomarkers could have a potential role in patient risk stratification and eventually offer the potential to allow the effect of conventional and emerging pharmacologic interventions with novel mechanisms of action as well.53
Correspondence: Dr P.W Serruys.
Prof. of Interventional Cardiology.
Thoraxcenter, Bd 406. Erasmus MC.
Dr Molewaterplein 40. 3015 GD Rotterdam. The Netherlands.
E-mail: p.w.j.c.serruys@erasmusmc.nl