There is sufficient evidence that type 2 diabetes mellitus (T2DM) increases cardiovascular morbidity and mortality. A recent study estimated that for every point increase in glycated hemoglobin (HbA1c), the relative risk of cardiovascular disease (CVD) increases by 18%.1 However, interventional studies have been inconclusive in demonstrating that optimized glycemic control equates to a reduction in CVD. This raises the importance of choosing agents according to their cardiovascular effect, independently of their effectiveness in reducing blood glucose levels. Over the last decade, various results have been reported on this topic: generally, they have shown that most antidiabetic agents have, at best, a neutral effect on cardiovascular protection. However, most of those results were obtained from subanalyses of clinical trials with a primary objective other than determination of cardiovascular safety.
In 2008, following the withdrawal of rosiglitazone from the market due to its apparent association with increased cardiovascular risk, there was somewhat of a turning point. As part of the regulatory process, the Food and Drug Administration (FDA) called for clinical trials specifically aimed at assessing the cardiovascular safety of all new antidiabetic medications coming onto the market,2 and deemed it unacceptable for the upper limit confidence interval of the cardiovascular risk ratio to exceed 1.8.2
Since then, there have been 6 published multicenter prospective randomized control trials assessing the cardiovascular safety of drugs used in the treatment of T2DM, which altogether included more than 58 000 participants. Three of these trials evaluated the dipeptidyl peptidase-4 (DPP-4) inhibitors saxagliptin (SAVOR-TIMI 53 trial3), alogliptin (EXAMINE trial4), and sitagliptin (TECOS5); 2 evaluated the peptide-like glucagon-1 receptor (GLP-1R) agonists lixisenatide (ELIXA trial6) and liraglutide (LEADER trial7); and 1 evaluated the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (EMPA-REG OUTCOME trial8). All the DPP-4 inhibitors studied and lixisenatide demonstrated noninferiority to placebo for cardiovascular morbidity and mortality, which was the primary outcome of these trials. However, both empagliflozin (EMPA-REG OUTCOME trial8) and, very recently, liraglutide (LEADER trial7) have shown superiority to placebo in reducing cardiovascular morbidity and mortality. These results are an important landmark and could change the clinical management of diabetic patients, given the prognostic significance of CVD in this patient group.
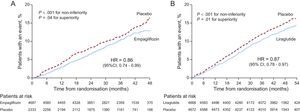
The EMPA-REG OUTCOME trial is the first clinical trial to demonstrate that an antidiabetic agent can reduce cardiovascular events.8 The trial studied 7020 patients with T2DM and established CVD, who were followed up over a mean period of 3.1 years. The primary outcome event was a composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke (MACE). Empagliflozin significantly reduced the risk of major adverse cardiovascular events (MACE) (hazard ratio [HR] = 0.86; 95% confidence interval [95%CI], 0.74-0.99; P = .04). However, several points warrant discussion. Firstly, the statistical significance obtained in MACE was due to cardiovascular mortality, which was reduced by 38% (HR = 0.62, P = .001) in the group treated with empagliflozin; there were no significant differences in the incidence of nonfatal myocardial infarction (a slight decrease) or nonfatal stroke (a slight increase). Secondly, there were no differences between the 2 doses used (10mg and 25mg); therefore, there was no demonstrable dose-response effect in terms of cardiovascular safety. Thirdly, empagliflozin reduced the rate of hospitalization for heart failure by 35% and had a beneficial effect on mortality from heart failure. Lastly, and perhaps most surprisingly, differences in the 2 treatment arms (empagliflozin versus placebo) became apparent very early on and became significant for the primary endpoint (MACE) at 3 months after starting treatment. Such rapid results suggest that the observed effect is down to a hemodynamic or metabolic, rather than antiarteriosclerotic, effect of empagliflozin.9,10
The decrease in systolic/diastolic blood pressure was approximately 5/2 mmHg and was maintained throughout the study. While this could have contributed to the reduction in cardiovascular events, it would not be expected to see such an effect until at least 1 year after starting treatment, rather than at 3 months. In addition, this slight reduction in blood pressure probably should have had a greater effect on stroke than on coronary events but, as mentioned, this was not the case. Therefore, it is unlikely that the reduction in blood pressure plays a significant role in the cardiovascular safety associated with empagliflozin. The diuretic effect of empagliflozin, through a reduction in intravascular volume (preload) and an improvement in arterial pressure and aortic stiffness (afterload), could improve ventricular function and myocardial oxygen demand. This could be an especially attractive option for patients with a reduced ejection fraction and heart failure. Measurement of B-type natriuretic peptide could be useful to confirm this hypothesis and identify the subgroup of patients that would benefit most from this treatment.9 Aside from these hemodynamic factors, it is worth mentioning that there is probably a metabolic factor inherent to moderate hyperketonemia that occurs with the increase in β-hydroxybutyrate in patients treated with SGLT2 inhibitors. β-hydroxybutyrate is the fuel used most by the myocardium and provides more energy, in the form of adenosine triphosphate, than other sources, and it is hypothesized that the slightly elevated concentration of this ketone has a cardioprotective effect.11 In addition, SGLT2 inhibitors have been demonstrated to increase the hematocrit, probably as a consequence of hemoconcentration caused by their diuretic effect. This increase in hematocrit could facilitate tissue release of oxygen in the myocardium, thus contributing to cardioprotection.11 The mechanisms involved in the glucose-lowering action of SGLT2 inhibitors and their pleiotropic effects have been reviewed recently.10
There are no published studies on cardiovascular safety with other SGLT2 inhibitors besides empagliflozin, therefore we cannot establish whether the effect observed with empagliflozin is specific to the molecule or an inherent class effect. Another area that will need to be addressed with ad hoc clinical trials is how to manage diuretic use in patients on treatment with SGLT2 inhibitors.
The LEADER trial7 included 9340 patients, of whom 81% had established CVD and/or renal failure. The mean follow-up was 3.8 years and the primary outcome event, as in the EMPA-REG OUTCOME trial, was the composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke. Treatment with liraglutide resulted in a 13% reduction in the primary outcome event (HR = 0.87; 95%CI, 0.78-0.97; P = .01 for superiority). There was a reduction in cardiovascular mortality, nonfatal stroke, nonfatal myocardial infarction, and hospitalization for heart failure, but only the reduction in cardiovascular mortality was statistically significant (22% reduction). Unlike the EMPA-REG OUTCOME trial, the effect of the drug took longer to become evident (Figure). In the LEADER trial, the differences between the placebo group and the liraglutide treatment group started to become evident after the first year of follow-up, indicating an effect on atheromatosis progression more so than a hemodynamic or metabolic effect.
Chronological differences in the beneficial effects of empagliflozin and of liraglutide on cardiovascular events (composite of cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke) in the EMPA-REG OUTCOME8 (A) and LEADER7 (B) trials. 95%CI, 95% confidence interval; HR, hazard ratio.
The presence of GLP-1R has been demonstrated in the myocardium and in vessels (endothelial and muscle cells). The mechanisms by which GLP-1R agonists exert beneficial effects directly on the cardiovascular system have recently been reviewed.12,13 In summary, GLP-1R agonists regulate blood pressure, heart rate, myocardial contractility, and tissue perfusion. They also reduce vascular inflammation and protect against oxidative stress. Furthermore, a recent experimental study demonstrated that exenatide reduces platelet activation and thrombus formation.14 Lastly, treatment with GLP-1R agonists is associated with a slight reduction in total cholesterol, low-density lipoprotein cholesterol, and triglycerides,15 but there is insufficient information to conclude that this slight improvement in lipid profile has a significant impact on cardiovascular events.
One question that remains unanswered is whether the effect observed with liraglutide is exclusive to this GLP-1R agonist or whether it is a class effect. As already mentioned, the ELIXA trial demonstrated noninferiority (neutral effect) compared with placebo in terms of cardiovascular safety. However, the trials on GLP-1R agonists are not comparable in terms of design and methodology: the ELIXA trial6 included 6068 patients with a history of myocardial infarction or hospitalization for unstable angina in the 6 months prior to study inclusion, who were randomized (placebo versus lixisenatide) and followed up for a mean of 25 months. This patient profile is distinct from that in the LEADER trial. Furthermore, the primary outcome analyzed in ELIXA was the composite of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina (the unstable angina criterion was not used in the LEADER trial). Therefore, although there are definite differences in the molecular structure and pharmacokinetics of liraglutide and lixisenatide, currently available data do not allow us to conclude that liraglutide is the only GLP-1R agonist with a beneficial cardiovascular effect in high-risk patients.
Currently, there are 11 ongoing clinical trials (6 with GLP-1R agonists, 3 with DPP-4 inhibitors and 2 with SGLT2 inhibitors), which altogether will include more than 80 000 patients. These could provide further answers, such as clarifying whether the reduction in cardiovascular events observed with empagliflozin and with liraglutide is a class effect or a specific effect of those particular drugs. It would also be very useful if these studies were to incorporate multivariable analyses to ascertain which variables independently influence the results obtained. Both the EMPA-REG OUTCOME trial and the LEADER trial used this type of statistical analysis.
Following the FDA recommendations on the cardiovascular safety of antidiabetic agents based on ischemic events could be problematic: it would be costly and may not be an efficient way to identify and evaluate other cardiovascular problems such as heart failure. Thus, from a cost-benefit perspective, it may be more effective to adopt a more individualized approach, performing cardiovascular safety studies targeted the specific questions that need to be answered, and only when there is a justifiable indication. For example, in the phase before or after authorization, if a drug appears to precipitate heart failure, then a clinical trial should be designed aimed specifically at addressing that issue.
Clearly, information on cardiovascular safety is essential for cardiologists when making treatment decisions, but we must take care to avoid placing the focus solely on cardiovascular safety and overlooking microvascular complications or, as has occurred in some studies, performing only a limited analysis with methodological designs that leave much to be desired. Both cardiologists and endocrinologists must not lose the holistic view of the patient. For instance, it would be highly irresponsible to use a drug that was safe from a cardiovascular perspective but that caused diabetic macular edema and led to blindness in a high percentage of the population. Another emerging example is that of cognitive impairment, in particular the prevalence of Alzheimer disease in patients with T2DM. Given the neuroprotective action of GLP-1 and the abundance of its receptors in the brain, clinical trials are underway to demonstrate whether GLP-1R agonists could prevent Alzheimer. If such trials were to demonstrate that they reduced cognitive impairment in the diabetic population, it would be inappropriate to not take this property into account when choosing an antidiabetic agent, especially for older patients with diabetes, who are at an increased risk of developing dementia. All of this offers a new perspective on prescribing antidiabetic therapy, moving toward the more personalized approach of precision medicine, albeit with some new complexities.
In short, the EMPA-REG OUTCOME and LEADER trials have started a revolution in drug choice for patients with T2DM, which will affect all professionals involved in their care. Specifically, it seems reasonable to prescribe these types of drugs to patients who have already had a cardiovascular event, while awaiting the results of trials that evaluate their cost-effectiveness. The results of such trials are likely to have an impact on the clinical guidelines on this subject, and we must remain mindful of this. It should be made clear that there is no evidence of a cardioprotective effect in patients who have not had a cardiovascular event or who are low risk. It will be particularly interesting to see whether the combination of empagliflozin and liraglutide has an additive or synergistic effect on the prevention of cardiovascular events. As the mechanisms of action appear to be completely different, it seems reasonable to hazard the possibility of synergy, but we must await the results of clinical trials on this subject. Although there remain many unanswered questions, we can be certain that there is an exciting future ahead that will require even closer collaboration between cardiologists and endocrinologists to optimize the treatment of DM, one of the major pandemics of the 21st century.
CONFLICTS OF INTERESTNone declared.