Nonfluoroscopic catheter ablation is feasible in most procedures. The aim of our registry was to evaluate the safety and feasibility of a zero-fluoroscopic approach to catheter ablation in several Spanish centers.
MethodsEleven centers prospectively included a minimum of 20 patients. Patients with an arrhythmic substrate deemed suitable by the operator for a zero-fluoroscopic approach throughout the procedure were recruited. Patients with intracardiac devices were not included. Attending electrophysiologists, fellows, and resident physicians participated in each procedure, as in usual care.
ResultsThe study included 247 patients. Ablation was performed in 235 patients (95.2%). In 2 patients, who were not included in the analysis, fluoroscopy was performed as the first-line treatment. The arrhythmic substrate was located in the right chambers in most of the procedures (231 of 233 [99.15%]). Fluoroscopy was used in 24 procedures (10.3%). Catheter ablation was successful in 96.4% of the procedures and severe complications occurred in 2 patients (0.85%). Two variables were related to the need for fluoroscopy: the performing center (minimum 0% vs maximum 30.3%; P=.001) and procedural failure (13% vs 2.4%; P<.05).
ConclusionsThe Spanish multicenter registry reveals that a zero-fluoroscopic approach is feasible in most right-sided catheter ablation procedures. Randomized trials are necessary to confirm the safety of this approach. The need for fluoroscopy was related to procedural failure, with significant differences among performing centers.
Keywords
The treatment of choice for most cardiac tachyarrhythmias is catheter ablation. Catheter movement within a patient's cardiovascular system is typically visualized using fluoroscopy. Unfortunately, ionizing radiation has adverse effects on both patients and staff. Some of these effects are serious, such as cancer and genetic mutation induction (stochastic effects),1 and can develop despite the judicious use of fluoroscopy and radiation protection clothing.1 Additionally, these garments can cause vertebral injuries that lead to invasive treatments and/or sick leave.2
Nonfluoroscopic intracardiac navigation systems (NFINSs) reduce the amount of fluoroscopy required for safe and successful ablation.3 However, there are differences between the methodology of an ablation procedure aiming to avoid fluoroscopy use (zero-fluoroscopy approach) and that of a procedure using a NFINS to merely reduce fluoroscopy use (minimal fluoroscopy approach). Data on zero-fluoroscopy ablation have been published by centers with extensive experience.4–14 However, there is little information on the results of centers with very different experience levels. The aim of this multicenter registry was to evaluate the results of zero-fluoroscopy ablation procedures in various Spanish centers with distinct experience levels.
METHODSThe 11 participating centers had variable experience with the use of mapping systems in zero-fluoroscopy procedures. Two centers had performed fewer than 10 such procedures before the registry started, whereas the other 9 had performed more than 10, although not all operators had conducted this type of procedure. Each center had to enroll a minimum of 20 patients but was free to enroll more until all the centers had enrolled the minimum number. Patients were prospectively enrolled. All staff members who typically took part in ablation procedures in each center could participate in the registry. Approval of the registry was first granted by the Ethics Committee of the Complejo Hospitalario Universitario of Granada (coordinating center) and then ratified by the other relevant committees. All patients provided written informed consent.
Inclusion CriteriaThe registry included patients with arrhythmic substrates who, according to the treating physician, could be managed with a completely nonfluoroscopic procedure from the beginning (zero-fluoroscopy approach). All substrates localized to the right heart (atrioventricular nodal reentrant tachycardia, accessory pathways, atrial tachycardia, and right ventricular outflow tract tachycardia) were considered, although other substrates could be included as long as they met the study criteria.
Exclusion CriteriaPatients without electrocardiographic evidence of clinical tachyarrhythmia were excluded, as well as those with intracardiac leads or an arrhythmic substrate requiring a transseptal or epicardial approach. If the presumptive diagnosis was not confirmed and the arrhythmic substrate to be addressed could not be treated without first-line fluoroscopy, the procedure was considered a protocol deviation and was omitted from the final analysis.
Ablation ProcedureThe entire procedure, from first puncture to catheter removal, had to have been performed without the support of first-line fluoroscopy. The Ensite-NavX™ system (St. Jude Medical; St. Paul, Minnesota, United States) was used in all procedures. Fluoroscopy use was at the discretionof the attending electrophysiologist; no patient's safety was jeopardized in an attempt to avoid its use. The reasons for fluoroscopy use were analyzed.
All centers performed the ablation according to their standard practice in terms of personnel training, number, access, and position of diagnostic catheters, and type and access of ablation catheters. The analysis included the identity of the operators of the electroanatomy system (technician, nurse, resident, electrophysiologist fellow, attending electrophysiologist), the diagnostic catheters, and the ablation catheter in each procedure.
The total procedure time was defined as the interval between the first puncture and catheter removal. Waiting time was not predefined. The recorded complications corresponded to those appearing during the hospitalization period; also analyzed were the rate of repeat ablations of a previously treated substrate. Recurrence was not considered to have occurred if there was no evidence of specific recurrence in the previously ablated substrate. The follow-up time was not predefined and was the standard duration for each center.
Statistical AnalysisCategorical variables are expressed as frequencies and percentages. The normality assumption of continuous variables was assessed with the Kolmogorov-Smirnov test. Variables following a normal distribution are expressed as mean ± standard deviation. The remaining variables are expressed as median [interquartile range]. Associations between categorical variables were assessed using the chi-square or Fisher exact test, as appropriate. Associations among quantitative variables were determined using Pearson or Spearman correlation, depending on whether the variables satisfied the normality condition. Comparisons of quantitative variables between the 2 groups were performed with the Student t test (normal variables) or with the nonparametric Mann-Whitney U test (nonnormal variables).
Differences with a probability of error less than 5% (P < .05) were considered significant. All analyses were performed using SPSS for Mac (version 20.1).
Analysis of the need for fluoroscopy and related variables was performed for procedures that did not use fluoroscopy as first-line treatment, after exclusion of patients who did not ultimately undergo ablation or whose substrate was ablated using first-line fluoroscopy.
RESULTSThis registry prospectively included 247 patients from November 2014 to September 2015. The time to inclusion of the minimum number of required patients (20 patients) varied (167 ± 11 [range, 51-296] days).
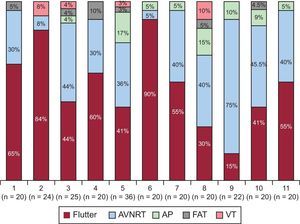
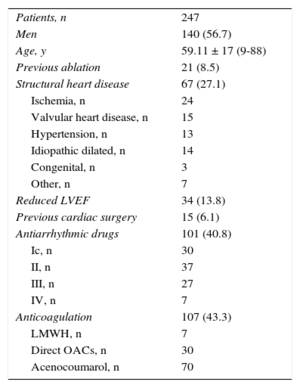
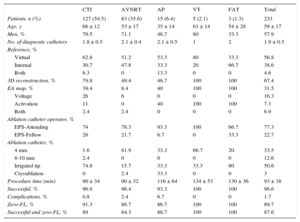
The patients’ baseline characteristics are shown in Table 1. In order of frequency, the arrhythmias prompting the ablation were common atrial flutter (n = 129, 52.2%), atrioventricular nodal reentrant tachycardia (AVNRT) (n = 88, 35.6%), accessory pathways (n = 17, 6.9%), focal right atrial tachycardia (n = 7, 2.8%), and right ventricular tachycardia (n = 6, 2.4%). This distribution varied among the participating centers (Figure 1).
Baselines Characteristics
| Patients, n | 247 |
| Men | 140 (56.7) |
| Age, y | 59.11 ± 17 (9-88) |
| Previous ablation | 21 (8.5) |
| Structural heart disease | 67 (27.1) |
| Ischemia, n | 24 |
| Valvular heart disease, n | 15 |
| Hypertension, n | 13 |
| Idiopathic dilated, n | 14 |
| Congenital, n | 3 |
| Other, n | 7 |
| Reduced LVEF | 34 (13.8) |
| Previous cardiac surgery | 15 (6.1) |
| Antiarrhythmic drugs | 101 (40.8) |
| Ic, n | 30 |
| II, n | 37 |
| III, n | 27 |
| IV, n | 7 |
| Anticoagulation | 107 (43.3) |
| LMWH, n | 7 |
| Direct OACs, n | 30 |
| Acenocoumarol, n | 70 |
LMWH, low-molecular-weight heparin; LVEF, left ventricular ejection fraction; OACs, oral anticoagulants.
Unless otherwise indicated, the data represent No. (%) or mean ± standard deviation (range).
Frequency distribution of the arrhythmic substrates prompting the electrophysiology study in each center; in parentheses, the total number of procedures in each center. AP, accessory pathway; AVNRT, atrioventricular nodal reentrant tachycardia; FAT, focal atrial tachycardia; VT, ventricular tachycardia.
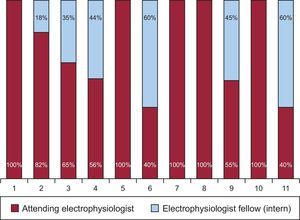
The operators of the diagnostic catheters were residents in 17.4% of the procedures, electrophysiologist fellows (interns) in 30.4%, and attending electrophysiologists in 52.2%. The operators of the ablation catheter (n = 27, 2.4 ± 1 per center) were interns in 22.7% of procedures (n = 7) and attending electrophysiologists in 77.3% (n = 20); this percentage varied according to the performing center (Figure 2) and the substrate being treated (Table 2).
Data on Ablation Procedures Using a First-line Zero-fluoroscopy Approach
| CTI | AVNRT | AP | VT | FAT | Total | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 127 (54.5) | 83 (35.6) | 15 (6.4) | 5 (2.1) | 3 (1.3) | 233 |
| Age, y | 66 ± 12 | 53 ± 17 | 35 ± 14 | 63 ± 14 | 54 ± 28 | 59 ± 17 |
| Men, % | 79.5 | 71.1 | 46.7 | 80 | 33.3 | 57.9 |
| No. of diagnostic catheters | 1.8 ± 0.5 | 2.1 ± 0.4 | 2.1 ± 0.5 | 1 | 2 | 1.9 ± 0.5 |
| Reference, % | ||||||
| Virtual | 62.8 | 51.2 | 53.3 | 80 | 33.3 | 56.8 |
| Internal | 30.7 | 47.8 | 33.3 | 20 | 66.7 | 38.6 |
| Both | 6.3 | 0 | 13.3 | 0 | 0 | 4.6 |
| 3D reconstruction, % | 79.8 | 49.4 | 46.7 | 100 | 100 | 67.4 |
| EA map, % | 39.4 | 8.4 | 40 | 100 | 100 | 31.5 |
| Voltage | 26 | 6 | 0 | 0 | 0 | 16.3 |
| Activation | 11 | 0 | 40 | 100 | 100 | 7.3 |
| Both | 2.4 | 2.4 | 0 | 0 | 0 | 6.9 |
| Ablation catheter operator, % | ||||||
| EPS-Attending | 74 | 78.3 | 93.3 | 100 | 66.7 | 77.3 |
| EPS-Fellow | 26 | 21.7 | 6.7 | 0 | 33.3 | 22.7 |
| Ablation catheter, % | ||||||
| 4 mm | 1.6 | 81.9 | 33.3 | 66.7 | 20 | 33.5 |
| 8-10 mm | 2.4 | 0 | 0 | 0 | 0 | 12.6 |
| Irrigated tip | 74.8 | 15.7 | 33.3 | 33.3 | 80 | 50.6 |
| Cryoablation | 0 | 2.4 | 33.3 | 0 | 0 | 3 |
| Procedure time (min) | 90 ± 34 | 90 ± 32 | 116 ± 64 | 134 ± 53 | 130 ± 36 | 93 ± 38 |
| Successful, % | 96.9 | 96.4 | 93.3 | 100 | 100 | 96.6 |
| Complications, % | 0.8 | 2.4 | 6.7 | 0 | 0 | 1.7 |
| Zero-FL, % | 91.3 | 86.7 | 86.7 | 100 | 100 | 89.7 |
| Successful and zero-FL, % | 89 | 84.3 | 86.7 | 100 | 100 | 87.6 |
AP, accessory pathway; AVNRT, atrioventricular nodal reentrant tachycardia; CTI, cavotricuspid isthmus; EA, electroanatomical; EPS, electrophysiological study; FAT, focal atrial tachycardia; FL, fluoroscopy; VT, ventricular tachycardia.
In total, 485 diagnostic catheters were used in the 247 procedures (1.96 ± 0.53 [1-4] catheters/procedure): 209 quadripolar catheters, 135 decapolar, 120 duodecapolar, 2 octopolar, 3 pentapolar, and 6 circular; 2 ablation catheters were used as diagnostic catheters (8 catheters were undefined). In most patients, femoral vein access was used; use of the brachial and subclavian veins was less frequent (44 and 13 patients, respectively).
Ablation was performed in 235 procedures and was not performed in 12 for various reasons: in 8 due to absence of the expected substrate, in 2 due to a left-sided flutter, in 1 due to mechanical block of the accessory pathway, and in 1 due to difficult localization of atrial tachycardia. Two patients with left-sided accessory pathways were excluded from the analysis because the presumptive diagnosis was not confirmed and, according to the criteria of the operator in charge, fluoroscopy was required as first-line treatment.
Finally, we analyzed 233 ablation procedures performed without the first-line use of fluoroscopy during the entire procedure (both diagnostic and therapeutic) (Table 2). Focal atrial tachycardias were located in the interatrial septum (n = 2) and the crista terminalis (n = 1). The locations of the accessory pathways were as follows: 7 right inferior, 4 right superior, and 2 right inferior paraseptal and 2 left free wall pathways. The procedure time was 93 ± 38 (31-255) minutes and the waiting time was 18 ± 9minutes. Fluoroscopy was necessary in 24 procedures (10.3%). The total required fluoroscopy time was 5990seconds (median, 120 [interquartile range, 22-372] [range, 9-1200]. Fluoroscopic assistance was required due to difficulties with venous access to the cardiac chambers (n = 8), with ablation catheter manipulation (n = 7), and with diagnostic catheter placement (n = 6) and due to displacement of the positional reference of the electroanatomical system with map movement (n = 3). Fluoroscopy was not used in any of the 12 procedures not ultimately requiring ablation.
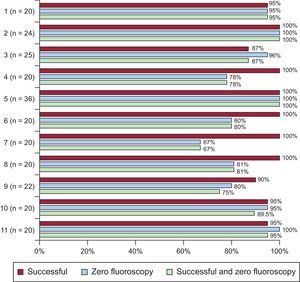
The percentage need for fluoroscopy ranged from 0% to 33.3% (P = .001) according to the performing center (Figure 3). Of the 2 centers that had performed less than 10 nonfluoroscopic procedures before the registry, 1 never required fluoroscopy and the other required it in just 20% of patients. In the ablation catheter procedures performed by an attending electrophysiologist, fluoroscopy was required in 10% (18 of 180) vs 9.4% (5 of 53) for those performed by an electrophysiologist fellow (no significant difference). There was no relationship between the number of procedures per center and the percentage of fluoroscopy use. The variables related to the need for fluoroscopy were the performing center (maximum, 33.3%; minimum, 0; P = .001) and procedural failure (13.0% vs 2.4%; P < .05). An association was found between the time (days) to the inclusion of the minimum number of patients (20 patients) and the percentage of fluoroscopy use (r = 0.69; P = .019).
A successful outcome was achieved in 96.5% of the 233 procedures. Success by arrhythmic substrate was as follows: 96.9% for ablation procedures of the cavotricuspid isthmus, 96.4% for those of the AVNRT, 93.3% for those of the accessory pathways, and 100% for those of ventricular tachycardia and focal atrial tachycardia. Thus, the percentage of ablation procedures achieving success and not requiring fluoroscopy during the entire procedure varied according to the arrhythmic substrate treated (Table 2). These results also varied among performing centers (Figure 3).
Complications occurred in 4 of the 247 patients (1.6%): 2 severe (1 vascular complication during ablation of the cavotricuspid isthmus and 1 atrioventricular block requiring a definitive pacemaker after an AVNRT ablation) and 2 transient (1 second-degree atrioventricular block during AVNRT ablation and 1 transient AH interval prolongation during cryoablation of a right inferior septal accessory pathway). Due to recurrence of the previously treated substrate, 4 of the 225 patients (2 flutters, 1 AVNRT, and 1 accessory pathway) underwent successful and fluoroscopy-free repeat ablation (1.8%).
DISCUSSIONThis is the multicenter registry of nonfluoroscopy-guided catheter ablation with the largest number of participating hospitals and centers to date. This registry shows that ablation of substrates located in right cardiac cavities guided exclusively by a NFINS is practically feasible in 90% of procedures, with success and complication rates similar to previously published standard rates.15
The use of NFINSs significantly reduces the radiation dose in substrates of different complexities.3 NFINS is now an indispensable first-line tool for the ablation of complex arrhythmic substrates that can drastically reduce the radiation dose.16 Randomized studies have also estimated a significant reduction in cancer risk in less complicated procedures performed with a NFINS.17,18 Thus, the greater the radiation dose reduction, the greater the beneficial effects. However, it is a little optimistic to expect the complete abolishment of radiation from the very beginning of the ablation of complex substrates (in addition to complication-free elimination of the arrhythmic substrate). Nonetheless, some authors have published their experience with nonfluoroscopic ablation of atrial fibrillation.7,9 It is likely that this goal (completely radiation-free ablation) will remain restricted to procedures tackling less complex substrates.
Various centers have published their experience with completely NFINS-guided catheter ablation in different substrates and patient groups.4–14 The bias of these studies is due to their reliance on single-center experiences, which is why the reported results are not generalizable to other centers with less experience. Accordingly, the experience of multicenter studies can provide more information on feasibility and safety.
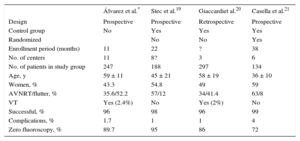
Three multicenter studies19–21 have been published (Table 3), with differences in design and type of patient included. The results of our registry (success and complications) are comparable to those published by these 3 studies (Table 3). Nonetheless, there are clear differences in the percentage of fluoroscopy-free procedures. In our analysis, the performing center and the procedural result were related to the need for fluoroscopy, but this analysis was not performed in the other 3 registries. In our group, only 2 centers had extensive experience with these procedures.8,10,14 It would seem obvious that the operator's experience and confidence with these procedures are key factors in the completion of a zero-fluoroscopy procedure, even when the procedure is successful. In our registry, there were no differences according to operator experience (attending electrophysiologist vs fellow). This might be because fluoroscopy use is at the discretion of the person in charge of the procedure (attending electrophysiologist) who, due to their higher confidence, could have directed the maneuvers of the electrophysiology fellow to achieve a zero-fluoroscopy procedure. In the other registries, the participation criteria of the operators varied; in the NO-PARTY study,18 the participating centers (6 in total) had acquired experience in previous years21; only 3 operators with extensive experience participated in another of the registries20 and, in the other, patients were treated with a zero-fluoroscopy approach only when the procedure could be performed by an experienced operator.18 There is no detailed information on the zero-fluoroscopy learning curve. In 1 study, a single operator performed all fluoroscopy-free ablation procedures (n = 60). This operator's learning curve comprised 17 nonconsecutive procedures during a 5-month period.13 Another registry reported that the learning curve comprised 10 procedures.19 We believe that a crucial factor affecting procedural confidence is the time that elapses while the experience is being accumulated. A long time interval between procedures probably impedes the acquisition of experience and, thus, confidence. Here, there was a direct association between the fluoroscopy percentage and the time required to enroll the minimum number of patients.
Comparison With Other Multicenter Registries
| Álvarez et al.* | Stec et al.19 | Giaccardiet al.20 | Casella et al.21 | |
|---|---|---|---|---|
| Design | Prospective | Prospective | Retrospective | Prospective |
| Control group | No | Yes | Yes | Yes |
| Randomized | No | No | Yes | |
| Enrollment period (months) | 11 | 22 | ? | 38 |
| No. of centers | 11 | 8? | 3 | 6 |
| No. of patients in study group | 247 | 188 | 297 | 134 |
| Age, y | 59 ± 11 | 45 ± 21 | 58 ± 19 | 36 ± 10 |
| Women, % | 43.3 | 54.8 | 49 | 59 |
| AVNRT/flutter, % | 35.6/52.2 | 57/12 | 34/41.4 | 63/8 |
| VT | Yes (2.4%) | No | Yes (2%) | No |
| Successful, % | 96 | 98 | 96 | 99 |
| Complications, % | 1.7 | 1 | 1 | 4 |
| Zero fluoroscopy, % | 89.7 | 95 | 86 | 72 |
AVNRT, atrioventricular nodal reentrant tachycardia; VT, ventricular tachycardia.
The substrate distribution was also different. In our registry, the percentage of patients with substrates located in the left cavities was minimal (0.8%). We decided to analyze those patients with arrhythmic substrates who were to be treated without fluoroscopy as a first-line approach; these substrates were predominantly located in right chambers. In our registry, some centers but not others used the zero-fluoroscopy method in the retroaortic approach for the ablation of accessory pathways. Other authors have published their experience with the ablation of left-sided substrates using additional imaging techniques (transesophageal and/or intracardiac echocardiography) to perform the transseptal puncture.7,12 This method is not necessarily the standard approach in electrophysiology laboratories and therefore its use was not presumed in this registry
The Ensite-NavX NFINS was used in all procedures because it permits monitoring of all catheters, both diagnostic and therapeutic, from their introduction into the patient's vascular system, which favors the effective execution of a zero-fluoroscopy approach. In addition, because it is an open system, different ablation catheters can be used without affecting the final objective. Nonetheless, other NFINSs also enable a zero-fluoroscopy approach.17,22
The safety of this procedure is supported by our results (success, complications), which are equivalent to those of other studies and the data from the Spanish Catheter Ablation Registry.15 Nonetheless, caution is required because nonfluoroscopic ablation is not complication free. One complete and 1 transient atrioventricular block out of 89 patients with AVNRT are a source of concern.
LimitationsThe absence of a control group reduced the statistical power of our results. Reduced fluoroscopy use has also been shown in previous studies, but our findings are not diminished by their similarity to those of other registries.
In some centers, the inclusion period was long, due to various prerequisites (human and material resources) for the inclusion of each patient. Consequently, some patients with treatable substrates may not have been included, although all patients meeting all requirements were indeed consecutively enrolled.
Even though the inclusion criteria permitted left-sided substrates, the researchers were only “comfortable” applying a zero-fluoroscopy approach to right-sided substrates. Left-sided substrates (accessory pathways) were only treated in 4 patients; 2 were omitted from the analysis because fluoroscopy was not the first-line treatment. Thus, our results on the ablation of these substrates are scarce.
Of the 2 centers with more previous experience with zero-fluoroscopy approaches, 1 included 36 patients and the other 20. Thus, these centers had a higher percentage of fluoroscopy-free procedures. If all of the centers had included more patients, this percentage would have been higher.
CONCLUSIONSOur multicenter registry shows that fluoroscopy-free ablation in procedures localized to the right cardiac cavities is feasible in 90% of standard procedures in unselected centers. Analysis is required of procedural safety in randomized studies. Fluoroscopy is more frequently used in unsuccessful ablation procedures. The need for fluoroscopy varies according to the center performing the ablation.
CONFLICTS OF INTERESTM. Álvarez and L. Tercedor have received consulting fees from St. Jude Medical.
- –
During a catheter ablation procedure, NFINSs significantly reduce the radiation dose to patients and staff.
- –
Fluoroscopy-free ablations can be performed in centers with extensive experience.
- –
The lower the amount of radiation absorbed, the lower the risk of stochastic effects.
- –
Data on fluoroscopy-free ablation in unselected centers and those with different experience levels.
- –
Operator experience and ablation outcome are associated with the likelihood of successful completion of radiation-free ablation procedures.
We would like to thank all technicians, residents, nurses, and electrophysiologists for their enthusiastic participation in this registry.
Complejo Hospitalario Universitario de Granada: Manuel Molina, Pablo Sánchez; Hospital San Juan de Alicante: José Moreno, Jesús Castillo; Hospital Basurto de Bilbao: José Ormaetxe, Jesús Martínez-Alday, Larraitz Gaztañaga; Hospital Juan Ramón Jiménez de Huelva: Juan Miguel Fernández; Hospital Clínico de Valencia: Ricardo Ruiz-Granell, Maite Izquierdo, Juan Miguel Sánchez, Ángel Martínez; Hospital Virgen de la Salud de Toledo: Miguel Ángel Arias, Alberto Puchol, Finn Akerstrom; Hospital General de Alicante: Santiago Heras, Alicia Ibáñez; Hospital Gregorio Marañón de Madrid: Pablo Ávila; Hospital Sant Pau de Barcelona: Xavier Viñolas, José Guerra; Hospital La Fe de Valencia: Óscar Cano, Ana Andrés, Pau Alonso.