Both atrial fibrillation (AF) and chronic kidney disease (CKD) are highly prevalent, especially with increasing age and associated comorbidities, such as hypertension, diabetes, heart failure, and vascular disease. The relationship between both AF and CKD seems to be bidirectional: CKD predisposes to AF while onset of AF seems to lead to progression of CKD. Stroke prevention is the cornerstone of AF management, and AF patients with CKD are at higher risk of stroke, mortality, cardiac events, and bleeding. Stroke prevention requires use of oral anticoagulants, which are either vitamin K antagonists (eg, warfarin), or the nonvitamin K antagonist oral anticoagulants (NOACs). While NOACs have been shown to be effective in mild-to-moderate renal dysfunction, there are a paucity of data regarding NOACs in severe and end-stage renal dysfunction. This review first discusses the evidence for NOACs in CKD. Second, we summarize the current knowledge regarding the efficacy and safety of NOACs to prevent AF-related stroke and systemic embolism in severe and end-stage renal disease.
Keywords
Both atrial fibrillation (AF) and chronic kidney disease (CKD) are highly prevalent, especially with increasing age and associated comorbidities, such as hypertension, diabetes, heart failure, and vascular disease. The relationship between both AF and CKD seems to be bidirectional: CKD predisposes to AF while onset of AF seems to lead to progression of CKD. Importantly, the concurrence of AF and CKD leads to an increased risk of thromboembolic complications including stroke, systemic thromboembolism, and myocardial infarction.1 Paradoxically however, CKD is itself a risk factor for bleeding.2
Stroke prevention is the cornerstone of AF management and requires use of oral anticoagulants (OAC), which are either vitamin K antagonists (VKA) (eg, warfarin), or the non-VKA oral anticoagulants (NOACs).3 While NOACs have been shown to be effective in mild-to-moderate renal dysfunction, there are a paucity of data regarding NOACs in severe and end-stage renal dysfunction (ESRD).
This review first discusses the evidence for NOACs in CKD. Second, we summarize the current knowledge regarding the efficacy and safety of NOACs to prevent AF-related stroke and systemic embolism in severe and end-stage renal disease.
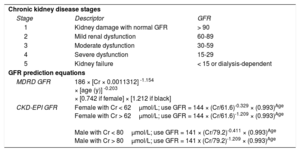
DEFINITIONS AND EPIDEMIOLOGICAL CONSIDERATIONSChronic kidney disease is classified into stages 1 to 5 by the Kidney Disease Improving Global Outcomes based glomerular filtration rate (GFR) or albuminuria present for > 3 months. The GFR may be estimated through use of the Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease-Epidemiology Collaboration group (CKD-EPI) equations4 (Table 1). Severe renal dysfunction implies GFR less than 30mL/min/1.73m2.5 There is variability in the definition of ESRD in clinical trials; however a suggested framework to diagnose ESRD is symptomatic uremia necessitating chronic (> 30 days) renal replacement therapy.6 Renal replacement therapy may be delivered via extracorporeal (hemodialysis) or paracorporeal (peritoneal dialysis) modalities.7
Chronic Kidney Disease Stages and Prediction of Glomerular Filtration Rate Equations
| Chronic kidney disease stages | ||
| Stage | Descriptor | GFR |
| 1 | Kidney damage with normal GFR | > 90 |
| 2 | Mild renal dysfunction | 60-89 |
| 3 | Moderate dysfunction | 30-59 |
| 4 | Severe dysfunction | 15-29 |
| 5 | Kidney failure | < 15 or dialysis-dependent |
| GFR prediction equations | ||
| MDRD GFR | 186 × [Cr × 0.0011312] -1.154 × [age (y)] -0.203 × [0.742 if female] × [1.212 if black] | |
| CKD-EPI GFR | Female with Cr < 62μmol/L; use GFR = 144 × (Cr/61.6)-0.329 × (0.993)Age Female with Cr > 62μmol/L; use GFR = 144 × (Cr/61.6)-1.209 × (0.993)Age Male with Cr < 80μmol/L; use GFR = 141 × (Cr/79.2)-0.411 × (0.993)Age Male with Cr > 80μmol/L; use GFR = 141 x (Cr/79.2)-1.209 × (0.993)Age | |
CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; Cr, creatinine; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
The prevalence of AF in ESRD ranges from 7% to 27% in different studies, which is 10- to 20-fold higher than in the general population.8 For example, in the Chronic Renal Insufficiency Cohort (CRIC) prospective study of 3267 patients with mild-to-moderate CKD (mean GFR 43.6 ± 13.4mL/min/1.73 m2) AF was present in 18% participants, suggesting that the processes underlying AF onset may occur early in the course of CKD.9
Atrial fibrillation may also contribute to CKD progression. A subgroup analysis of the CRIC study found a higher rate of progression to ESRD in CKD patients with incident AF (11.8/100 person-years) compared with CKD patients that did not develop AF (3.4/100 person-years) over a mean follow-up of 5.9 years.10 Thus, the relationship between AF and renal dysfunction is bidirectional. Of note, the mortality associated with incident AF has been shown to be higher in patients with CKD (survival 63.4%-68.3% at 12 months) than without CKD (79.3% survival at 12 months).11
SEARCH STRATEGYFor this review, a search of published studies was performed using bibliographic databases (PubMed, Medline, Scopus, Cochrane database) and scanning reference lists from published articles. Search terms used were: “NOAC”, “chronic kidney disease”, “atrial fibrillation”, “dialysis”, “apixaban”, “rivaroxaban”, “edoxaban”, and “dabigatran”. Emphasis was placed on randomized controlled trials (RCTs) and original data.
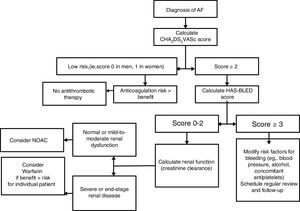
STROKE, BLEEDING RISK, AND THROMBOPROPHYLAXIS IN ATRIAL FIBRILLATIONAtrial fibrillation increases the risk of stroke in AF, but this risk is not homogeneous, and it depends on various risk factors. The more common and validated stroke risk factors have been used to formulate stroke risk stratification scores, such as the CHA2DS2VASc score.12 Of note, stroke risk is not static and regular reassessment is needed, given the increasing age and incident risk factors over time.13
Recognizing that CKD is additive to stroke risk, some studies have proposed the addition of CKD or renal surrogates (eg, proteinuria) to risk scores, such as the ATRIA score14 or the R2CHADS2 score.15 However, other studies have not shown an additive value for stroke prediction by considering CKD.16–18 This is perhaps unsurprising since CKD is highly associated with the individual components of the CHA2DS2VASc score. The CHA2DS2VASc score has been studied in dialysis patients, where it has been validated for stroke prediction.13
Chronic kidney disease also predisposes to an increased risk of bleeding. Although numerous bleeding risk factors have been described including various biomarkers, bleeding and stroke risk factors are often similar, thus stroke and bleeding rates track each other. In this sense, the HAS-BLED score, which incorporates the more common validated bleeding risk factors, has been proposed to assess bleeding risk.19 The appropriate use of the HAS-BLED score is to draw attention to the modifiable bleeding risk factors, and to ‘flag up’ the high bleeding risk patients for more frequent review and follow-up.20 Of note, an approach simply focused on modifiable bleeding risk factors alone is an inferior strategy to HAS-BLED for bleeding risk prediction.13,21,22
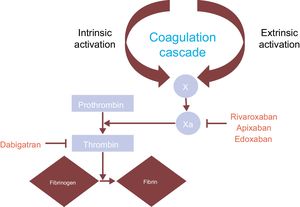
Thromboprophylaxis in AF requires oral anticoagulants, which can be well-managed VKA (eg, warfarin) or NOACs (eg, apixaban, rivaroxaban, edoxaban, and dabigatran).3 Although warfarin and NOACs act on the coagulation pathway they differ in their mechanism of action. Warfarin inhibits the vitamin K-dependent clotting factors II, VII, IX and X to influence the international normalized ratio (INR): a low INR increases clotting risk whereas a high INR increases bleeding risk. By contrast, the NOACs target individual clotting proteins: apixaban, rivaroxaban, and edoxaban directly inhibit factor X and dabigatran is a direct thrombin inhibitor23 (Figure 1).
A prospective study of 565 patients on warfarin demonstrated that individuals with severe renal dysfunction (GFR < 30mL/min per 1.73kg/m2) required lower warfarin doses and were less time in therapeutic range (TTR). Moreover, the incidence rate of major hemorrhage was greater in severe renal dysfunction (30.5 per 100 patient-years) compared with mild (6.2 per 100 patient-years) and moderate (8.3 per 100 patient-years) renal dysfunction.24 In addition, warfarin use is also complicated by multiple drug and food interactions and a reduction in vitamin K-dependent matrix G1a protein that results in increased vascular calcification.25 Warfarin-related nephropathy resulting from glomerular hemorrhage and tubular obstruction by red cell casts is also seen more frequently in patients with CKD.26
Nonvitamin K antagonist oral anticoagulants have changed the landscape for stroke prevention in AF, although regional differences are evident.27 In contrast to warfarin, NOACs have fewer drug and diet interactions, have a rapid onset of action and shorter half-life, and do not require regular laboratory monitoring.28 The short half-life means that adherence and compliance is crucial, and patients need education and counselling.29
When glomerular filtration is impaired, clearance of NOACs is decreased, thus their plasma half-life is prolonged. This may result in increased total drug exposure or area under the curve (AUC), which increases the risk of bleeding complications.30 Dabigatran has significant renal clearance (80% renal excreted), with lower renal excretion seen for edoxaban (50%), rivaroxaban (33%) and apixaban (27%).31 There are limited data for NOACs in severe and ESRD (Cockcroft-Gault creatinine clearance [CrCl] < 25-30mL/min) as these patients were excluded from the phase 3 randomized trials32 (Table 2).
New Oral Anticoagulants: Renal Clearance and Dosing
| NOAC | Target | Oral bio-availability (%) | Renal clearance of absorbed drug | Tmax (h) | Half-life(h) | Dose in normal renal function (ESC) | Dose in normal renal function (FDA) | Dose in moderate renal impairment (ESC) | Dose in moderate renal impairment (FDA) | Dose in severe renal impairment (ESC) | Dose in severe renal impairment (FDA) | Dose in ESRD/ dialysis (ESC) | Dose in ESRD/dialysis (FDA) | Dialyzable |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban | Factor Xa | 50 | 27% | 3-4 | 8-13 | 5mg twice daily | 5mg twice daily | 5mg twice daily | 2.5-5mg twice daily* | No | 2.5-5mg twice daily | No | 2.5-5mg twice daily | No |
| Rivaroxaban | Factor Xa | 80-100 | 30% | 2-4 | 5-13 | 20mg once daily | 20mg once daily | 15mg daily | 15mg daily (CrCl 30-50 mL/min) | No | 15mg daily | No | No | No |
| Edoxaban | Factor Xa | 62 | 50% | 1-2 | 10-14 | 60mg daily | 60mg daily | 30mg daily | 60mg daily (CrCl 15-50 mL/min) | No | 30mg daily | No | No | No |
| Dabigatran | Thrombin | 6.5 | > 80% | 2-6 | 12-14 | 150mg twice daily | 150mg twice daily | 110mg twice daily | 150mg twice daily (CrCl > 30 mL/min) | No | 75mg twice daily | No | No | Yes |
CrCl, creatinine clearance; ESC, European Society of Cardiology; ESRD, end-stage renal disease; FDA, Food and Drug Administration; NOAC, nonvitamin K antagonist oral anticoagulant.
The phase III trials supporting the use of NOACs to prevent thromboembolism in AF were performed with specific NOAC dosing and patient exclusion criteria. Patients with CrCl < 30mL/min (dabigatran, rivaroxaban, and edoxaban) or CrCl < 25mL/min (apixaban) were excluded from the pivotal clinical trials.33–36
The European Society of Cardiology recommendations for moderate CKD (GFR, 30-59mL/min) are based on secondary analyses of phase 3 NOAC trials.37 By contrast, the Food and Drug Administration (FDA) in the United States have approved reduced dose dabigatran 75mg twice daily, apixaban 5mg twice daily (apixaban 2.5mg twice daily if ≥ 80 years old ≤ 60kg) and rivaroxaban 15mg twice daily in patients with CrCl 15 to 29mL/min mainly based on pharmacological modelling data for CrCl 15 to 29mL/min (Table 3).38-42
New Oral Anticoagulants: Key Trials and Renal Exclusion Criteria
| New oral anticoagulant | Trial | Dose | Study population | Renal exclusion criteria | Method | Key conclusion–risk of stroke and systemic embolism versus warfarin | Risk of bleeding relative to warfarin |
|---|---|---|---|---|---|---|---|
| Apixaban vs warfarin | ARISTOTLE39 | Apixaban 2.5-5mg twice daily | 18 201 patients | CrCl < 30 mL/min | Randomized, double-blind trial | Lower risk | Lower risk |
| Rivaroxaban vs warfarin | ROCKET-AF40 | Rivaroxaban 20mg daily | 14 264 patients | Creatinine > 221 mmol/L or CrCl < 25 mL/min | Randomized double-blind trial | Noninferior | Similar risk |
| Edoxaban vs warfarin | ENGAGE-AF TIMI41 | Edoxaban 30-60mg | 21 105 patients | CrCl < 30 mL/min | Randomized, double blind trial | Noninferior | Lower risk |
| Dabigatran vs warfarin | RE-LY42 | Dabigatran 110-150mg | 18 113 patients | CrCl < 30 mL/min | Randomized noninferiority trial | Dabigatran 150mg lower risk | Dabigatran 150mg similar risk |
CrCl, creatinine clearance.
The ARISTOTLE (Apixaban for Reduction In Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial compared apixaban at a dose of 5mg twice daily with warfarin (target INR 2-3). The rate of stroke or systemic embolism was significantly lower in patients treated with apixaban (1.27% per year) than with warfarin (1.60% per year). Use of apixaban was also associated with a 31% reduction in major bleeding and an 11% reduction in all-cause mortality compared with warfarin. Of note, individuals with a serum creatinine level of > 2.5mg per deciliter (221μmol per liter) or calculated CrCl of < 25mL/min were excluded from the trial.39
Hohnloser et al.43 evaluated the outcomes of the ARISTOTLE trial in relation to renal function. Apixaban was more effective than warfarin to prevent stroke and systemic embolism in all 3 distinct subgroups of renal function (GFR > 80, GFR > 50-80, GFR ≤ 50 and CrCl > 25mL/min). Patients with the most impaired renal function (GFR ≤ 50) benefitted from the greatest reduction in major bleeding on apixaban.
Renal function may worsen over time in patients with AF but apixaban was associated with a lower risk of stroke and major bleeding than warfarin even in patients with pronounced worsening (GFR deterioration > 20%) of renal function.44
RivaroxabanThe ROCKET-AF (Rivaroxaban Once Daily Oral Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism) trial compared rivaroxaban with warfarin (target INR 2-3) in patients with CrCl > 30mL/min. Significantly, the dose of rivaroxaban was reduced to 15mg daily in patients with a CrCl of 30-49mL/min (rivaroxaban 20mg daily for CrCl > 50mL/min). ROCKET-AF demonstrated that rivaroxaban is noninferior to warfarin in the prevention of stroke and systemic thromboembolism with no difference in the rate of major bleeding.40
Subgroup analysis of rivaroxaban in moderate renal dysfunction (CrCl 30-49mL/min) demonstrated noninferiority to warfarin in the prevention of stroke and systemic thromboembolism consistent with the initial trial findings. There were fewer fatal bleeds on rivaroxaban (0.28% per 100 patient-years) than warfarin (0.74% per 100 patient-years) in moderate renal dysfunction.45 In another study, outcomes for patients with AF and moderate-to-severe CKD on warfarin or rivaroxaban (matched based on demographics, comorbidities, stroke and bleeding risk factors and medications) again showed no significant difference in rates of thromboembolism or major bleeding.46
Kubitza et al.47 examined the pharmacokinetics of a single dose of rivaroxaban 10mg in 24 individuals with renal dysfunction compared with 8 patients with normal renal function. Progressive renal dysfunction increased rivaroxaban drug exposure (AUC increase 44% mild: 52% moderate: 64% severe, when compared with normal renal function) and increased the percentage inhibition of Factor Xa (50% mild: 86% moderate: 100% severe compared with normal renal function). There was a 26% increase in peak concentration of rivaroxaban in 8 patients with a mean CrCl of 43mL/minute.30,47
EdoxabanEdoxaban was evaluated in the ENGAGE-AF TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial. Patients were randomized to receive warfarin (target INR 2-3), edoxaban 60mg once daily, or edoxaban 30mg once daily. The dose of edoxaban was halved if the CrCl was 30 to 50mL/min, a body weight ≤ 60kg, or use of verapamil or quinidine. Patients with CrCl < 30mL/min were excluded from the trial. Both edoxaban regimens were noninferior to warfarin in the prevention of stroke or systemic embolus and in an on-treatment analysis edoxaban 60mg led to a 21% reduction in stroke or systemic embolism. Edoxaban was dose-dependently associated with fewer major bleeding events: 20% and 53% fewer events for edoxaban 60mg once daily and edoxaban 30mg once daily, respectively, compared with warfarin.37,41,48
An evaluation of the ENGAGE-AF TIMI 48 considered patient subgroups defined by CrCl 30 to 50mL/min, > 50 to 95mL/min, and > 95mL/min. In patients with moderate renal dysfunction (CrCl 30-50mL/min) edoxaban 30mg once daily remained comparable to warfarin for prevention of thromboembolism with fewer major bleeding events. Although there were fewer thromboembolic events overall in patients with normal renal function (> 95mL/min) edoxaban 60mg once daily had numerically lower efficacy than warfarin to prevent thromboembolic events in this subgroup, putatively due to optimal renal clearance resulting in subtherapeutic dosing.49
In a multicenter study over a 12-week period, there was no significant difference in bleeding events in patients with severe renal impairment (CrCl 15-30mL/min) treated with edoxaban 15mg daily compared with those with normal or mild renal impairment (CrCl > 50mL/min) treated with edoxaban 30mg or 60mg once daily.50 The FDA has approved use of lower dose edoxaban 30mg once daily in patients with CrCl 15 to 50mL/min.32 This dose reduction is based on pharmacokinetic data of 1281 patients receiving edoxaban whereby lower renal function conferred a higher risk of bleeding.51
DabigatranThe RE-LY study randomized 18 113 patients to receive warfarin or dabigatran 110mg or 150mg twice daily over a 2-year follow-up period. Dabigatran 150mg twice daily led to significantly fewer stroke and systemic embolism events (1.11% per year) compared with warfarin (1.69% per year). Dabigatran 110mg twice daily led to 20% fewer major bleeding events but was noninferior to warfarin in preventing stroke and systemic embolism (1.53% per year).42 A RE-LY subgroup analysis study used baseline creatinine values to estimate GFR based on Cockcroft-Gault and CKD-EPI equations to divide the study population into 3 groups: GFR ≥ 80mL/min, GFR 50 to 80mL/min and GFR < 50mL/min. Dabigatran 150mg twice daily remained superior and dabigatran 110mg twice daily was noninferior to warfarin in preventing stroke and systemic embolism across the spectrum of renal function regardless of the method of GFR calculation. Further, dabigatran 110mg twice daily had fewer bleeding events and dabigatran 150mg twice daily had similar bleeding events to warfarin across the subgroups of renal function. In patients with GFR > 80 estimated by the CKD-EPI equation, both dabigatran doses had lower rates of major bleeding.52
In severe CKD, the FDA recommends dabigatran 75mg twice daily although clinical evidence to support this dosing is lacking. The dose reduction is based on a pharmacokinetic study performed through obtaining blood and urine samples from 23 patients with different levels of renal dysfunction receiving a single oral dose of dabigatran. Compared with patients with normal kidney function, the AUC was shown to be 1.5-, 3.2- and 6.3-fold higher in participants with mild, moderate, and severe renal impairment respectively.53
COMPARING THE NOACs IN CHRONIC KIDNEY DISEASEIn a meta-analysis of 13 878 AF patients with moderate CKD, the NOACs were compared through assessing surface under the cumulative ranking (SUCRA) curves. Dabigatran 150mg twice daily was the most efficacious (SUCRA 0.96) followed by apixaban, rivaroxaban, and edoxaban (SUCRA 0.67; 0.53; 0.51 respectively). Apixaban (SUCRA 0.84) and edoxaban (SUCRA 0.61) had the most favorable safety profile.54
NOACs IN END-STAGE RENAL DYSFUNCTIONAtrial fibrillation in dialysis patients poses a complex clinical dilemma. The highest incidence of AF is seen in hemodialysis patients (15.1%) compared with nonend-stage CKD (9.6%) and in normal renal function (2.6%).2 End-stage renal dysfunction increases bleeding risk secondary to platelet dysfunction (disturbance of platelet α-granules, fibrinogen fragments binding to the glycoprotein (GP) IIb/IIIa receptor on platelets, uremic toxins and abnormal calcium mobilization all contribute to reduced aggregation and adhesion of platelets).34
There are no RCT investigating the use of NOACs in severe and end-stage renal disease. Even ESRD patients were excluded from the RCTs of warfarin for stroke prevention in AF. Nonetheless, data on warfarin use comes from observational cohorts, where good-quality anticoagulation control is crucial to minimize the risks of stroke and bleeding in these high-risk patients.55,56 By contrast, observational data from North America, which generally do not report TTR, suggest that ischemic stroke is not reduced but serious bleeding significantly increased in patients with end-stage renal failure (ESRF).57,58
For example, Harel et al. performed a meta-analysis of 14 observational studies examining the use of warfarin in the prevention of ischemic stroke in ESRD. There was no significant difference in ischemic stroke or intracranial hemorrhage in patients treated with warfarin. The key limitations of this meta-analysis were that the definition of stroke and bleeding varied between studies (for instance transient ischemic attack was accounted for as a primary outcome in certain studies but not recorded in others). The quality of warfarin anticoagulation (ie, TTR) was also not accounted for in the included studies.59,60
Alternatively, a prospective study of warfarin treatment in ESRD (median TTR 54%) showed that higher TTR reduced the risk of bleeding,55 implying that warfarin use in ESRD must be accompanied by adequate INR surveillance for maximum benefit. In a Danish registry study over a 15-year study period, patients with AF were subdivided into those with nonend-stage CKD (11 128 patients) or on renal replacement therapy (1728). There was a lower risk of death on warfarin therapy in both nonend-stage CKD and renal replacement therapy (hazard ratio [HR], 0.64; 95% confidence interval [95%CI], 0.60 -0.69 and HR, 0.85; 95%CI, 0.72-0.99 respectively). In nonend-stage CKD, a lower composite risk of fatal stroke and bleeding (HR, 0.71; 95%CI, 0.57-0.88) was observed.61
The clearance of drugs during hemodialysis is enhanced for small molecules (< 1500 daltons) and intravascular unbound drugs (low volume of distribution) as they pass easily through the dialysis membrane.30 Given these considerations, dabigatran is known to be the only dialyzable NOAC. Surprisingly, registries of ESRF patients report the use of NOACs in these patients. Indeed, data from the US Renal Data System show that NOAC use over warfarin increased from 0.16% to 29.16% (P-for-trend < .001) between October 2010 and December 2015.62
One study has directly compared bleeding rates for patients with AF and ESRD on hemodialysis and initiated on rivaroxaban, dabigatran, or warfarin. The highest rate of major bleeding was for ESRD patients treated with dabigatran (83.1 events per 100 patient-years) and rivaroxaban (68.4 events per 100 patient-years) compared with warfarin (35.9 events per 100 patient-years). Furthermore, mortality in patients taking dabigatran (19.2 deaths per 100 patient-years) was higher than in those on rivaroxaban (16.2 deaths per 100 patient-years) and warfarin (10.2 deaths per 100 patient-years) raising concern about the use of dabigatran in ESRD.63
Ongoing studies may further elucidate the use of NOACs on ESRD patients. The RENAL-AF NCT02942407 trial (Renal Haemodialysis Patients Allocated Apixaban Versus Warfarin in AF) is randomizing 762 patients to receive 2.5 to 5mg twice-daily apixaban or warfarin to measure outcomes of major bleeding episodes, stroke, and mortality over a 15 month period (completion May 2019). The AXADIA trial is a prospective, multicenter study in Germany that has enrolled 222 patients to examine the safety of apixaban vs the VKA phenprocoumon in AF patients on hemodialysis (completion April 2019).38 Future studies should further consider differences in the efficacy and safety of patients maintained on hemodialysis vs peritoneal dialysis as the excretion of NOACs may vary between these renal replacement therapies.
ApixabanIn a small parallel group study of 8 hemodialysis patients, a single dose of 5mg apixaban postdialysis resulted in 36% higher drug exposure (AUC) but no increase in peak serum concentration (Cmax) compared with patients with normal renal function. The finding that apixaban was well tolerated in this study of ESRD contributed to FDA-approval of apixaban 5mg twice daily in severe renal dysfunction and ESRD (reduced dose apixaban 2.5mg twice daily in ≥ 80 years old ≤ 60kg).30,64
A separate pharmacokinetic study suggests that apixaban 2.5mg twice daily in ESRD leads to drug exposure comparable to standard dose apixaban 5mg twice daily in patients with normal renal function whereas standard dose apixaban in ESRD leads to supratherapeutic levels.65
Stanton et al. retrospectively assessed 146 patients with severe renal impairment (CrCl < 25mL/min) or ESRD (on hemodialysis and peritoneal dialysis) who received either apixaban or warfarin. There were fewer major bleeding events in patients taking apixaban (9.6%) compared with warfarin (17.8%) and fewer composite-bleeding episodes (major bleeding, clinically relevant nonmajor bleeding, and minor bleeding) in the apixaban arm (27.4%) than the warfarin arm (21.9%) although these results were not statistically significant. The occurrence of ischemic stroke was similar in both groups. Apixaban was proposed to be safe alternative to warfarin in severe and end-stage renal disease in this study.66
In contrast, a case study has described the use of lower dose apixaban 2.5mg twice a day in an ESRD patient resulting in high peak/trough antifactor Xa levels and a resultant gastrointestinal bleed.67 Another study of 30 ESRD patients on maintenance hemodialysis who were treated with apixaban had a 13.3% incidence of major bleeding leading the authors to recommend caution in the prescription of apixaban for dialysis patients.68
RivaroxabanRivaroxaban is not dialyzable as it is highly protein bound (> 90%).69 Indeed, the AUC when rivaroxaban was given before hemodialysis was only 5% lower than when rivaroxaban was given after hemodialysis, suggesting minimal impact of hemodialysis on rivaroxaban pharmacokinetics.70 In a study of 18 chronic hemodialysis patients, administration of 10mg rivaroxaban led to drug exposure (AUC) comparable to 20mg rivaroxaban in patients with normal renal function. There was no significant accumulation with daily dosing.71
Indeed, Dias et al.70 showed that a 15mg dose of rivaroxaban led to 56% higher AUC in ESRD patients than in healthy controls. Taken together, these data would suggest requirement for rivaroxaban dose reduction in ESRD.
EdoxabanEdoxaban is not recommended in patients with CrCl < 15mL/min. In 1 study, 10 hemodialysis patients were randomized to receive 15mg edoxaban pre- and posthemodialysis. There was a nonsignificant decrease in AUC when edoxaban was given predialysis, suggesting that hemodialysis does not effectively remove edoxaban from the bloodstream. Although edoxaban was well tolerated by ESRD patients in this study, limitations are that only a single dose of edoxaban was given, follow-up time was very short and the patient cohort was small.72
DabigatranDabigatran has the highest renal clearance of all NOACs (80%) and is the only NOAC that may be removed by dialysis. In 1 case report of dabigatran dialysis for life-threatening bleeding there was a rebound increase in drug level during the elimination process possibly due to a high volume of distribution.73
CLINICAL CONSIDERATIONSIn the clinical environment, we must endeavor to use CrCl in the estimation of renal function to decide an appropriate anticoagulation strategy as it reflects the clinical trials most closely. Indeed, previous studies have shown a disparity in GFR estimation when the Cockcroft-Gault, CKD-EPI and MDRD equations are used.52
Patients prescribed NOACs should also have their renal function monitored to ensure that they are consistently prescribed the correct dose. For instance, a study in primary care has shown that annual renal function monitoring identifies patients who are over- or undercoagulated.74 Furthermore, compliance with renal function monitoring has been shown to increase the likelihood of adequate NOAC dosing at 1-year follow-up.75
We must additionally consider adjusting NOAC doses in acute-on-chronic kidney injury. In an observational study of 162 patients with concomitant AF and heart failure, the measured fluctuation in kidney function merited dosage adjustment (44%, 35%, 29% of patients on dabigatran, rivaroxaban and apixaban, respectively, required dose adjustment).76
CONCLUSIONThe decision to anticoagulate patients with concomitant AF and CKD depends on CKD stage, given the fine balance between the prevention of thromboembolism and excess bleeding especially in ESRF (Figure 2). In moderate CKD, the available data indicate NOACs are at least noninferior to warfarin in the prevention of stroke and systemic embolism with similar to superior safety profile. In severe renal failure (GFR 15-20mL), the use of NOACs is not routinely recommended as the pivotal trials excluded these patients. The EHRA practical guide generally favors the use of warfarin in this group of patients.48 Although the FDA has approved use of reduced dose NOACS in severe renal impairment, this is based on pharmacokinetic studies rather than prospective clinical trials.
An individualized approach is required to evaluate the risk vs benefit of anticoagulation in patients with AF and ESRD as there is evidence for and against its use. There are no RCT exploring the use of NOACSs in ESRD. Of note, the largest available head-to-head trial of outcomes in patients taking dabigatran, rivaroxaban or warfarin raised concern regarding the use of NOACs (in particular dabigatran) in hemodialysis due to increased bleeding risk.77 Thus, at present warfarin has more supportive evidence for its use in AF patients with ESRD to prevent stroke and systemic thromboembolism.
CONFLICTS OF INTERESTG.Y.H. Lip is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo; he declares not directly receiving any personal fees derived from these activities.
.