PCSK9 inhibitors (PCSK9i) are safe and effective lipid-lowering drugs. Their main limitation is their high cost. The aim of this study was to estimate the number of patients eligible for treatment with PCSK9i according to distinct published criteria.
MethodsData were obtained from the Information System for the Development of Research in Primary Care. Included patients were equal to or older than 18 years and had at least 1 low-density lipoprotein cholesterol measurement recorded between 2006 and 2014 (n = 2 500 907). An indication for treatment with PCSK9i was assigned according to the following guidelines: National Health System, Spanish Society of Arteriosclerosis, Spanish Society of Cardiology, National Institute for Health and Care Excellence, and the European Society of Cardiology/European Atherosclerosis Society Task Force. Lipid-lowering treatment was defined as optimized if it reduced low-density lipoprotein levels by ≥ 50% and adherence was > 80%.
ResultsAmong the Spanish population aged 18 years or older, the number of possible candidates to receive PCSK9i in an optimal lipid-lowering treatment scenario ranged from 0.1% to 1.7%, depending on the guideline considered. The subgroup of patients with the highest proportion of potential candidates consisted of patients with familial hypercholesterolemia, and the subgroup with the highest absolute number consisted of patients in secondary cardiovascular prevention.
ConclusionsThe number of candidates to receive PCSK9i in conditions of real-world clinical practice is high and varies widely depending on the recommendations of distinct scientific societies.
Keywords
Cardiovascular disease (CVD) is the leading cause of premature death and disability in Europe.1 A recent meta-analysis showed that a greater reduction in low-density lipoprotein cholesterol (LDL-C) was associated with greater cardiovascular benefit.2 Only 25% of patients with CVD3 and 3.4% of those with familial hypercholesterolemia (FH)4 achieve their lipid targets. Furthermore, around 5% to 20% of patients treated in real-world clinical practice and around 2% in randomized clinical trials have statin intolerance.5 Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are drugs that can reduce LDL-C by up to 65% and have a good safety profile.6 The FOURIER study7 demonstrated that adding evolocumab to statin treatment reduced the relative risk of the primary outcome of the study by 15%. In the coming months, the ODYSSEY Outcomes trial8 will help clarify the role of alirocumab in patients with coronary artery disease.
Nonetheless, the cost of PCSK9 inhibitors remains a considerable limitation to their more widespread use. Such factors have led the Ministry for Health, Social Services and Equality,9,10 the SEA (Spanish Society of Arteriosclerosis),11 the SEC (Spanish Society of Cardiology),12 NICE (National Institute for Health and Care Excellence),13,14 and the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) Task Force15 to establish indications for use.
The aim of this study was to estimate the number and type of patients who are candidates for receiving PCSK9 inhibitors in this country, according to different indication criteria, using a population database of 2 500 907 patients.
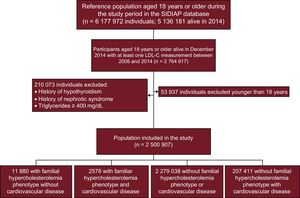
METHODSThis was an observational study that used the Information System for the Development of Research in Primary Care (SIDIAP, by its initials in Spanish), a clinical database of anonymous longitudinal registries of 6 177 972 patients between 2006 and 2014. SIDIAP includes information on the clinical activity of 3414 physicians from 274 primary care centers of the Catalan Institute for Health, a public entity providing health care cover to 85% of the population in Catalonia.16 It includes diagnoses (International Classification of Disease [ICD-10]), information on hospital discharge (ICD-9/10), laboratory findings, and medications dispensed by pharmacies. The validity of SIDIAP data for studying CVD epidemiology has been documented.17 The Clinical Research and Ethics Committee of the Institut Universitari d’Investigació en Atenció Primària (University Institute for Research in Primary Care) approved the study.
The study included all SIDIAP entries for individuals alive and aged ≥ 18 years in December 2014 with at least 1 LDL-C measurement between 2006 and 2014. Patients with a history of hypothyroidism, nephrotic syndrome, or baseline triglycerides ≥ 400 mg/dL were excluded.
VariablesParticipants were considered to be receiving lipid-lowering therapy if there was at least 1 recorded dispensing of a statin or ezetimibe from a pharmacy in the 6 months prior to LDL-C measurement, and untreated if there was no such record. To calculate the baseline value prior to treatment initiation in patients on lipid-lowering therapy, we used an algorithm with 10 possible imputations as per the methodology described by Jorgensen et al.18 (). Imputations were pooled according to Rubin's rules, in order to correct the existing variability in each imputation. A sensitivity analysis was performed from the results, carrying out 20 imputations as well as a separate analysis of those individuals with complete data. Treatment adherence was calculated using the medication possession ratio: the proportion of days in a 6-month period that are covered by the lipid-lowering treatment recorded as dispensed by the pharmacy. Lipid-lowering therapies were classified according to their capacity for LDL-C reduction: low-intensity, < 30% reduction; moderate, 30% to 50%; high, 50-60%; and very high, > 60% ().
Lipid-lowering therapy was considered optimized when the lipid-lowering intensity was ≥ 50% and adherence was > 80%.
The FH phenotype was defined according to the previously defined LDL-C cutoff points for the adult Spanish population adjusted for age: ≥ 18 to 30 years, > 230 mg/dL; 31 to 39 years, > 238 mg/dL; 40 to 48 years, > 260 mg/dL, and > 49 years, > 255 mg/dL.19
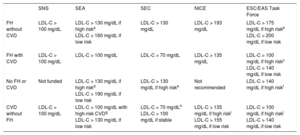
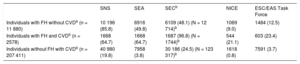
The diagnosis of CVD (peripheral arterial disease, coronary disease, ischemic stroke, and the presence of diabetes mellitus, hypertension, hypercholesterolemia or smoking) was determined based on the ICD-10 or ICD-9 recorded in the primary care and hospital registers. Table 1 shows the criteria used for defining candidates for PCSK9i according to the different organizations–the SNS (National Health System),9,10 the SEA,11 the SEC,12 NICE,13,14 and the ESC/EAS Task Force.15 Only indications funded by the SNS were included.9,10 The number of patients who were PCSK9i candidates in real life and in an optimized treatment scenario was calculated, applying the reduction in LDL-C observed in the optimized treatment patients to all patients. We also calculated the total percentage of patients who were PCSK9i candidates in the study population and in the Spanish population in a hypothetical situation involving a 50% LDL-C reduction from baseline.
Criteria From the SNS, SEA, SEC, NICE and ESC/EAS Task Force for the Use of PCSK9 Inhibitors.
| SNS | SEA | SEC | NICE | ESC/EAS Task Force | |
|---|---|---|---|---|---|
| FH without CVD | LDL-C > 100 mg/dL | LDL-C > 130 mg/dL if high riska LDL-C > 160 mg/dL if low risk | LDL-C > 130 mg/dL | LDL-C > 193 mg/dL | LDL-C > 175 mg/dL if high riskb LDL-C > 200 mg/dL if low risk |
| FH with CVD | LDL-C > 100 mg/dL | LDL-C > 100 mg/dL | LDL-C > 70 mg/dL | LDL-C > 135 mg/dL | LDL-C > 100 mg/dL if high riskc LDL-C > 140 mg/dL if low risk |
| No FH or CVD | Not funded | LDL-C > 130 mg/dL if high riskd LDL-C > 190 mg/dL if low risk | LDL-C > 130 mg/dL if high riske | Not recommended | LDL-C > 140 mg/dL if high riskf |
| CVD without FH | LDL-C > 100 mg/dL | LDL-C > 100 mg/dL with high-risk CVDg LDL-C > 130 mg/dL if low risk | LDL-C < 70 mg/dLh LDL-C > 100 mg/dL if stable | LDL-C > 135 mg/dL if high riski LDL-C > 155 mg/dL if low risk | LDL-C > 100 mg/dL if high riskj LDL-C > 140 mg/dL if low risk |
CVD, cardiovascular disease; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; NICE, National Institute for Health and Care Excellence; PCSK9 proprotein convertase subtilisin/kexin type 9; SEA, Spanish Society of Arteriosclerosis; SEC, Spanish Society of Cardiology; SNS, national health system.
SEA high-risk with FH in primary prevention: patients older than 40 years with other cardiovascular risk factors (hypertension, diabetes mellitus, smoking).
ESC/EAS Task Force high risk FH in primary prevention: patients with arterial hypertension or diabetes mellitus.
ESC/EAS Task Force high risk FH in secondary prevention: if progressive CVD (repeat events or requiring repeat revascularization).
SEA high risk in primary prevention without FH: presence of diabetes mellitus plus 2 cardiovascular risk factors or albuminuria or estimated glomerular filtration rate < 45 mL/min/1.73 m2.
SEC high risk in primary prevention without FH: type 1 or 2 diabetes mellitus with end organ damage, estimated glomerular filtration rate < 60 mL/min/1.73 m2 or SCORE > 10%.
ESC/EAS Task Force high risk in primary prevention without FH: type 2 diabetes mellitus with end organ damage or other risk factor.
SEA high risk in secondary prevention without FH: if unstable or progressive CVD (repeated events or requiring repeat revascularization) or presence of diabetes mellitus.
SEC high risk in secondary prevention without FH: unstable, relapsing (repeated events or requiring repeat revascularization) or recent (12 months) CVD.
Results are reported as percentage (categorical variables) and mean (continuous variables). In patients on lipid treatment with no available pretreatment LDL-C value, we used an algorithm with 10 possible imputations according to the methods described by Jorgensen et al.18 to estimate the pretreatment value. The variables included in the imputation model were age, sex, dose and type of lipid-lowering agent, and treatment adherence (). For the projection to a national scale of PCSK9i candidates, the prevalence of CVD was estimated based on the population diagnosed/population assigned in the SIDIAP database (independently of whether the patients had a recorded blood test); FH prevalence was extrapolated from the prevalence observed in our study. Statistical analysis was carried out using R-software.
RESULTSThere were 2 764 917 people who had at least 1 recorded LDL-C measurement. Of those, 2 500 907 patients met all the inclusion criteria. Figure 1 shows a flow diagram of the study. We identified 14 458 patients with an FH phenotype and 207 411 with CVD.
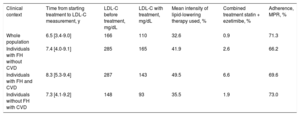
In the whole study population, 43.3% of the individuals on treatment did not have pretreatment LDL-C values available and therefore imputed values were used. The mean LDL-C before treatment was 166mg/dL and the mean observed LDL-C (treated and untreated) was 110mg/dL. The mean intensity of lipid-lowering therapy used was 32.6% and the calculated treatment adherence showed a medication possession ratio of 71.3% (Table 2).
Time Between Starting Lipid-lowering Treatment and LDL-C Measurement, LDL-C Values Before and During Treatment, Mean Lipid-lowering Intensity, Percentage of Combined Therapy (Statin + Ezetimibe) and Mean Adherence for the Whole Population and in Each Subgroup.
| Clinical context | Time from starting treatment to LDL-C measurement, y | LDL-C before treatment, mg/dL | LDL-C with treatment, mg/dL | Mean intensity of lipid-lowering therapy used, % | Combined treatment statin + ezetimibe, % | Adherence, MPR, % |
|---|---|---|---|---|---|---|
| Whole population | 6.5 [3.4-9.0] | 166 | 110 | 32.6 | 0.9 | 71.3 |
| Individuals with FH without CVD | 7.4 [4.0-9.1] | 285 | 165 | 41.9 | 2.6 | 66.2 |
| Individuals with FH and CVD | 8.3 [5.3-9.4] | 287 | 143 | 49.5 | 6.6 | 69.6 |
| Individuals without FH with CVD | 7.3 [4.1-9.2] | 148 | 93 | 35.5 | 1.9 | 73.0 |
CVD, cardiovascular disease; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; MPR, medication possession ratio.
Unless otherwise indicated, values are expressed as median [interquartile range].
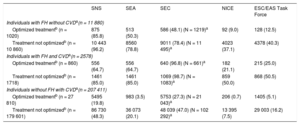
The number of candidates, according to the different criteria, is shown by patient subgroup and by treatment in Table 3.
Number and Percentage of Patients Who Are Candidates for PCSK9 Inhibitors in the Study Population in Conditions of Real-life Clinical Practice With and Without Optimized Lipid-lowering Therapy According to the Different Criteria.
| SNS | SEA | SEC | NICE | ESC/EAS Task Force | |
|---|---|---|---|---|---|
| Individuals with FH without CVDa(n = 11 880) | |||||
| Optimized treatmentb (n = 1020) | 875 (85.8) | 513 (50.3) | 586 (48.1) (N = 1219)a | 92 (9.0) | 128 (12.5) |
| Treatment not optimizedb (n = 10 860) | 10 443 (96.2) | 8560 (78.8) | 9011 (78.4) (N = 11 495)a | 4023 (37.1) | 4378 (40.3) |
| Individuals with FH and CVDa(n = 2578) | |||||
| Optimized treatmentb (n = 860) | 556 (64.7) | 556 (64.7) | 640 (96.8) (N = 661)a | 182 (21.1) | 215 (25.0) |
| Treatment not optimizedb (n = 1718) | 1461 (85.0) | 1461 (85.0) | 1069 (98.7) (N = 1083)a | 859 (50.0) | 868 (50.5) |
| Individuals without FH with CVDa(n = 207 411) | |||||
| Optimized treatmentb (n = 27 810) | 5495 (19.8) | 983 (3.5) | 5753 (27.3) (N = 21 043)a | 206 (0.7) | 1405 (5.1) |
| Treatment not optimizedb (n = 179 601) | 86 730 (48.3) | 36 073 (20.1) | 48 039 (47.0) (N = 102 292)a | 13 395 (7.5) | 29 003 (16.2) |
CVD, cardiovascular disease; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; FH, familial hypercholesterolemia; MPR, medication possession ratio; NICE, National Institute for Health and Care Excellence; PCSK9, proprotein convertase subtilisin/kexin type 9; SEA, Spanish Arteriosclerosis Society; SEC, Spanish Society of Cardiology; SNS, National health systems.
Values are expressed as No. (%).
Table 4 shows the number of candidate patients in an optimized treatment scenario. Approximately half of those who were PCSK9i candidates had an FH phenotype according to the SEA11 and NICE13,14 criteria. According to the ESC/EAS Task Force15 and to a greater extent the SNS9,10 and SEC12 criteria, more than 75% of the candidates were patients in secondary prevention.
Number and Percentage of Patients Who Are Candidates for PCSK9 Inhibitors in the Study Population in the Different Subgroups With Optimized Lipid-lowering Therapya According to the Different Criteria.
| SNS | SEA | SECb | NICE | ESC/EAS Task Force | |
|---|---|---|---|---|---|
| Individuals with FH without CVDb (n = 11 880) | 10 196 (85.8) | 6916 (49.8) | 6109 (48.1) (N = 12 714)b | 1069 (9.0) | 1484 (12.5) |
| Individuals with FH and CVDb (n = 2578) | 1668 (64.7) | 1668 (64.7) | 1687 (96.8) (N = 1744)b | 544 (21.1) | 603 (23.4) |
| Individuals without FH with CVDb (n = 207 411) | 40 980 (19.8) | 7958 (3.8) | 30 186 (24.5) (N = 123 317)b | 1618 (0.8) | 7591 (3.7) |
CVD, cardiovascular disease; ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; FH, familial hypercholesterolemia; MPR, medication possession ratio; NICE, National Institute for Health and Care Excellence; PCSK9, proprotein convertase subtilisin/kexin type 9; SEA, Spanish Arteriosclerosis Society; SEC, Spanish Society of Cardiology; SNS, National health systems.
Values are expressed as No. (%).
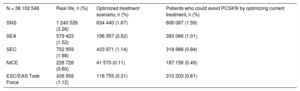
To estimate the number of PCSK9i candidates in the Spanish population aged ≥ 18 years, the prevalence of CVD observed in the assigned population in the whole SIDIAP data set was 6.2%. The prevalence of FH was 0.53%. In an optimized treatment scenario, the SNS9,10 criteria were those that indicated most PCSK9i candidates, at 1.7% of the Spanish population aged 18 years or older. The NICE13,14 criteria were the most restrictive, at 0.1% (Table 5).
Total Percentage of Patients Who Are Candidates for PCSK9 Inhibitors in the Spanish Population in Real-life and in the Optimized Treatment Scenario* and Number of Patients Who Could Avoid PCSK9 Inhibitor Treatment With Treatment Optimization.
| N = 38 102 546 | Real life, n (%) | Optimized treatment scenario, n (%) | Patients who could avoid PCSK9i by optimizing current treatment, n (%) |
|---|---|---|---|
| SNS | 1 240 526 (3.26) | 634 440 (1.67) | 606 087 (1.59) |
| SEA | 579 423 (1.52) | 196 357 (0.52) | 383 066 (1.01) |
| SEC | 752 959 (1.98) | 433 971 (1.14) | 318 988 (0.84) |
| NICE | 228 726 (0.60) | 41 570 (0.11) | 187 156 (0.49) |
| ESC/EAS Task Force | 426 958 (1.12) | 116 755 (0.31) | 310 203 (0.81) |
ESC/EAS, European Society of Cardiology/European Atherosclerosis Society; MPR, medication possession ratio; NICE, National Institute for Health and Care Excellence; PCSK9, proprotein convertase subtilisin/kexin type 9; SEA, Spanish Arteriosclerosis Society; SEC, Spanish Society of Cardiology; SNS, National health systems.
Optimization of lipid-lowering therapy resulted in a very substantial reduction in the number of candidates for treatment, by as much as half of the candidates for most of the criteria, being highest in the case of the SNS9,10 criteria (which went from 3.26% to 1.67% of the population) and lowest in the case of the NICE13,14 criteria (which went from 0.60% to 0.11% of the population) (Table 5).
In the hypothetical situation of a 50% reduction in LDL-C from baseline, the group of patients who are candidates for PCSK9 inhibitors was reduced considerably compared with the scenario based on LDL-C reductions observed in real-life clinical practice, between 0.89% according to SNS and 0.04% for NICE ().
Figure 2 shows the distribution of the population according to the LDL-C values in conditions of real-life clinical practice. LDL-C values > 130mg/dL were present in 77.4% of patients with FH but not CVD, in 44.9% of patients with FH and CVD, and in 17.2% of patients with CVD but not FH. Figure 3 shows the same distribution, but includes only patients who received optimized treatment. In this situation, LDL-C values > 130mg/dL were present in 51.4% of patients with FH but not CVD, 25.2% of patients with FH and CVD, and 3.3% of patients with CVD but not FH. In conditions of real-life clinical practice, in patients with CVD but not FH, 44.5% were above 100mg/dL and 81.7% were above 70mg/dL.
The sensitivity analysis of the results with 20 imputations showed no significant differences in the outcomes, population definitions, LDL-C values, or the percentage of PCSK9i candidates (). The analysis with complete data showed lower LDL-C values and a lower percentage of PCSK9i candidates ().
DISCUSSIONThis study used a database from real-life clinical practice in Spain to analyze the number of patients who were candidates for receiving PCSK9 inhibitors when different indication criteria were applied. Considerable differences were found in the type and number of candidates among the different criteria analyzed, ranging from 0.1% to 1.7% of the population aged ≥ 18 years. The required LDL-C target in each set of criteria largely explains these differences. Optimization of lipid-lowering therapy would considerably reduce the number of candidates. If optimization achieved a 50% LDL-C reduction from baseline, this reduction in candidate number would be very substantial.
In the subgroup of patients with FH phenotype in primary prevention, the number of patients who would be candidates after optimizing treatment was around 9% for NICE13,14 and 86%for SNS9,10; for the subgroup with FH in secondary prevention, it would be between 21% for NICE13,14 and 97% for SEC,12 and in patients with CVD but not FH, between 0.8% for NICE13,14 and 20% for SNS.9,10 The percentage of candidate patients in the population with FH according to the ESC/EAS Task Force15 criteria obtained in our study (12% in primary prevention and 23% in secondary prevention) is in line with the published figures from the FH registry from the SEA.20 A large part of the differences between criteria, especially in patients with FH in primary prevention, is due to the LDL-C cutoff for the treatment indication, and there are considerable increases if this is moved from 130 to 100mg/dL or 100 to 70mg/dL. 63.8% of patients with CVD but not FH have LDL-C values between 70 and 130mg/dL. In our population, moving from an LDL-C cutoff of 100 to 70mg/dL meant doubling the number of patients who are candidates (Figures 2 and 3). In the FOURIER study, 67% of patients on treatment with evolocumab achieved LDL-C levels < 40mg/dL and 42% achieved < 25mg/dL, and the absolute risk of CVD decreased from 11.3% to 9.8%.7 It should be noted that, for the same LDL-C value, the risk of CVD is up to 4 times higher in patients with FH than in those without FH.21 It is essential to determine the optimal LDL-C cutoff and the criteria for poor prognosis for PCSK9i indication in each patient subgroup so that such recommendations can be publicized. The SAFEHEART investigators have developed the first equation that can help improve prediction of CV risk in FH.22 Recent, diffuse, coronary artery disease that is progressive or cannot be revascularized indicates a poor prognosis in patients in secondary prevention.23
The type of patients also varies between the different criteria. Most patients with FH would be treated, if applying the SNS,9,10 SEA,11 and especially the SEC12 criteria, which focus on patients with FH and coronary disease. The criteria that relate more to patients with CVD without FH are the SEC12 and ESC/EAS Task Force15 criteria.
Another consideration is that the impact of cholesterol differs between coronary disease and other manifestations of CVD.24 The recommendations of the Spanish Society of Cardiology12 are exclusively for patients with coronary disease.
Another aspect to consider is the lack of studies on the cardiovascular benefit associated with dramatic reductions in LDL-C for patients over 75 years old in primary prevention.25 None of the guidelines mention potential age limits.
A recent meta-analysis put lipid control in the Spanish population at around 15% in secondary prevention and up to 65% in primary prevention, and detected–as in our study–underuse of high-intensity treatment and of combined use with ezetimibe.26 One of the biggest challenges in optimizing the rational use of PCSK9 inhibitors will be to avoid the over-diagnosis of statin intolerance.5 The use of electronic systems in decision-making could improve the number of patients with good lipid control.27 In the present study, when we simulated a scenario with optimized lipid treatment, the percentage of patients who were candidates for PCSK9 inhibitors according to the SEC12 criteria was reduced 1.7-fold.
If LDL-C levels were reduced by 50% from baseline in all patients, the percentage of candidates according to the SEC criteria would be reduced 2.7-fold ().
In a recent study, Cannon et al.28 created a simulation model for PCSK9i candidates in a USA population with CVD but not FH, based on successive adjustments in the dose and intensity of lipid-lowering therapy and with a target LDL-C < 70mg/dL. They estimated that 14% would be candidates, lower than the 24.5% estimated in our population according to the SEC13 criteria, which also set a target of LDL-C < 70mg/dL. These differences could be mostly explained by the simulation method used. In the same article they discuss different simulated scenarios and the percentage of PCSK9i candidates varies from 6% to 28%. The simulation scenario is based on the effects observed in real-life clinical practice in optimized patients, and in our opinion, this scenario is more realistic than assuming that the effect would be optimal and linear in all patients. In our alternative scenario with a 50% LDL-C reduction (), applying the same criteria, the percentage of candidates would be 10.9%.
Given that the prevalence of the FH phenotype in our setting is close to 0.5%29 and that it requires long-term treatment and has an onset at a young age, the direct impact of PCSK9 inhibitors on the public health system could be significant. To date, cost-effectiveness studies have had mixed results. A recent study found that the 5-year number needed to treat with PCSK9 inhibitors to prevent 1 cardiovascular event in patients at very high risk with an LDL-C target < 70mg/dL would be around 50.30 Another study showed that adding evolocumab to treatment with statins and ezetimibe in patients with FH may be a cost-effective measure.31 A recent study in the United States concluded that treatment with PCSK9 inhibitors would need to be around 70% cheaper to be cost-effective.32 A Norwegian study estimated that PCSK9 inhibitors would be cost-effective only as secondary prevention in older patients at very high risk.33 The only study in a Spanish population published to date showed that evolocumab may be cost-effective in patients with FH and patients in secondary prevention, with an incremental cost-effectiveness ratio of 30 893 euros and within the limits set as cost-effective in the Spanish population.34 However, we will have to wait for future cost-effectiveness studies on the results of the FOURIER7 and ODYSSEY Outcomes8 trials. Future studies to stratify risk within each subgroup will also be necessary.
Strengths and limitationsThe strengths of the study include the sample size and the approach based on real-world data. Another is that the population is representative, as shown by the analysis of complex patients: there was a lower percentage of PCSK9i candidates, as only younger patients who had started treatment more recently would have been included ().
As limitations, we must mention that the population studied included all individuals who had had an LDL-C test in an 8-year period, which represents approximately 57% of the general population aged 18 years or older and 70% of those aged 45 years or older. Therefore, we cannot rule out selection bias, especially in younger patients. In addition, the diagnostic method based on the FH phenotype may have overestimated its real prevalence, in particular because of patients with polygenic hypercholesterolemia, although they would also be candidates for PCSK9 inhibitors. Another limitation is that we did not have data on lipoprotein(a) or family history of CVD. In this study, it was not possible to evaluate what the possible impact of statin intolerance would be on PCSK9i prescribing. A further aspect that should be considered is the underdiagnosis of nonadherence to lipid-lowering treatment, as adherence was measured only by pharmacy dispensing of medications.
CONCLUSIONSThe number of potential candidates for PCSK9 inhibitors in conditions of real-life clinical practice is very high and varies substantially depending on the recommendations of the different scientific societies, at between 0.1% and 1.7% of the Spanish population aged ≥ 18 years. These differences are largely explained bytThe LDL-C target required in each set of criteria. The type of patients also varies between the different criteria. The subgroup with the highest percentage of potential candidates is composed of patients with FH and CVD, although in absolute terms the group with most candidates consists of patients in secondary prevention without FH. An intensive lipid-lowering treatment, with high-dose statins and combination therapy with ezetimibe, would considerably reduce the number of PCSK9i candidates.
FUNDINGMinistry of Economy, via the Instituto de Salud Carlos III (Cardiovascular Research Network-HERACLES Program RD12/0042 and redIAPP [Red de Investigación en Actividades Preventivas y Promoción de la Salud] RD12/0007) and the European Regional Development Fund, CIBER de Enfermedades Cardiovasculares. The government of Catalonia via the Agència de Gestió Ajuts Universitaris de Recerca (2014 SGR 240) and (2014 SGR 902) and via the Agència de Qualitat i Avaluació Sanitàries de Catalunya, Pla Estratègic de Recerca i Innovació en Salut (SLT002/16/00145).
CONFLICTS OF INTERESTN. Plana has given presentations funded by Alexion, Amgen, Ferrer, MSD, and Sanofi and attended scientific meetings funded by Amgen and Rovi. L. Masana has given presentations funded by Amgen, MSD, and Sanofi. À. Vila has given presentations funded by Ferrer, Sanofi, and Esteve and attended scientific meetings funded by Amgen and Ferrer.
- –
In patients with high or very high cardiovascular risk, there are unmet needs in lipid-lowering treatment.
- –
The approval of the new PCSK9 inhibitors is a promising step in lipid-lowering therapy.
- –
In our setting, there are different guidelines from the various scientific societies on the indications for PCSK9 inhibitors.
- –
The real impact of PCSK9 inhibitors in conditions of real-life clinical practice according to the different guidelines is unknown.
- –
An overwiew of the degree of control and use of lipid-lowering therapies for patients with high cardiovascular risk in conditions of real-life clinical practice.
- –
The proportion of the population and type of patients with an indication for PCSK9 inhibitors according to the different criteria of the various scientific societies and organizations.