Infective endocarditis (IE) is a rare disease that is difficult to diagnose, its treatment is complex and expensive, and mortality is high (around 20%). In the field of IE, the most recent changes are epidemiological and include an increased number of cases of IE in patients with prosthetic valves and intracardiac devices and in the population receiving health care, an increased number of cases of Staphylococcus aureus IE, and changes in antibiotic sensitivity patterns with a particular impact on vancomycin.1–3 Infective endocarditis requires prolonged hospitalization and resource-intensive treatment because antibiotic therapy, intensive care, and surgical treatment form part of the therapeutic approach.
Between 40% and 50% of patients require surgical treatment in the acute phase. In the year when IE is diagnosed, mortality is 30% with or without intervention. Mortality is high among certain groups, such as patients with prosthetic valves and especially those with intracranial hemorrhage.4
Early diagnosis and treatment can improve outcomes. Low suspicion of IE can delay effective treatment and increase mortality.5 In our experience, an appropriate therapeutic approach has a mean delay of 27 days when the patient is referred from another institution.
The treatment of IE is a paradigm of collaboration. Multidisciplinary teams (MDT) dedicated to IE have pioneered the organization of tertiary hospital care devoted to the disease.6,7 In the last decade, the International Collaboration in Endocarditis group has had a strong impact on understanding IE.8 The British Heart Valve Society has recommended the collaboration of specialists in valvular heart disease and IE9 and recent reports have supported this model.10
This article describes recommendations for the organization of an IE-MDT from the perspective of 30 years’ experience of the Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona with the aim of making this experience useful to the Spanish National Health System.
PRIOR CONSIDERATIONSInfective endocarditis is a medical-surgical disease in which surgical treatment is a part of the therapeutic process rather than a result of the failure of medical treatment. The care of patients with IE should be offered by an MDT comprising specialists with shared interests.
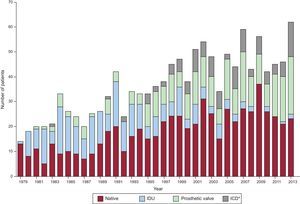
HISTORIC CONSIDERATIONSSince 1979, the organization and development of the Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona has been paralleled by developments in infectious disease, cardiology, microbiology, pathology, and cardiovascular surgery services. Figure 1 shows the annual distribution of cases of IE at our hospital since 1979 (1256 patients). Basic achievements have included the establishment of a database dedicated to IE, the commencement of the cardiovascular surgery service in 1979, the creation of a cardiovascular tissue bank (cryopreserved heart valves and arteries) in 1989, the introduction of transesophageal echocardiography in 1991, and the establishment of an electronic medical records system accessible through the hospital intranet in 1999.
Follow-up has been performed by specialists in infectious disease, cardiology, and cardiovascular surgery since 1986. The storage of pathogenic strains was organized at the microbiological biobank in 1993. The experimental endocarditis laboratory has been in operation since 1993, and the Working Group on Infective Endocarditis of the Hospital Clínic de Barcelona has maintained weekly meetings on IE since 1994.
STRUCTURE AND ORGANIZATION IN TERTIARY HOSPITALSThe benefits of an MDT are undeniable. Infective endocarditis should be treated in tertiary centers with an experienced cardiovascular surgery service.6 An MDT should comprise:
- •
A specialist in infectious diseases.
- •
A microbiologist.
- •
A specialist in valvular heart disease.
- •
An echocardiologist with extensive experience in the interpretation of valvular disease.
- •
A cardiovascular surgeon.
- •
A specialist in pathology.
- •
A specialist in outpatient parenteral antibiotic treatment (OPAT).
Tertiary hospitals also have the following services available: anesthesiology and resuscitation, diagnostic imaging, nuclear medicine, nephrology, neurology, neurosurgery, orthopedics and traumatology, and hemotherapy and blood management. The complexity of IE justifies the use of tertiary care.
Accumulated experience suggests the need for a home-care team for the management and follow-up of OPAT.11
The infectious disease service analyzes daily blood cultures at the hospital. If the results of blood cultures are positive, the action to be taken will be decided by consensus with the service that ordered the test.
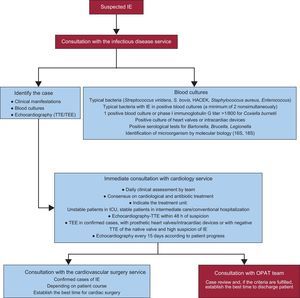
Overview of the ProcessThe treatment process of patients with IE is complex and involves several steps that commence when there is diagnostic suspicion of IE. The initial index of suspicion should be high, since the diagnosis of IE is often delayed due to the non-specificity of the symptoms.5 The initial step is to contact the infectious disease service, once there is clinical suspicion of IE or when blood culture results are positive from samples sent to the microbiology laboratory for various reasons. Once there is suspicion of IE and the criteria for IE have been confirmed, the cardiology and cardiovascular surgery services are contacted to form a consensus on which antibiotic and cardiological therapy to administer and to establish the indications for and timing of surgical intervention if applicable. Figure 2 shows this process.
Care process of patients with infective endocarditis admitted to hospital with a cardiovascular surgery service. HACEK, Haemophilus parainfluenzae, H. aphrophilus, H. paraphrophilus, H. influenzae, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae, and K. denitrificans; ICU, intensive care unit; IE, infective endocarditis; OPAT, outpatient parenteral antibiotic therapy; TEE, transesophageal echocardiography, TTE, transthoracic echocardiography.
Given that the process of treating IE is complex and involves an MDT, each case must be reviewed and policies for common action established. It is therefore recommended that the medical-surgical team hold weekly meetings to discuss patients who have been admitted, patients referred from other institutions, and patients attended in the emergency department. This meeting should confirm medical-surgical decisions and assess patients for OPAT. The team should also oversee the quality control process and analyze the postmortem study of any patients who died.
STRUCTURE AND ORGANIZATION IN HOSPITALS WITHOUT SURGICAL SUPPORTThe care of patients with IE is often difficult and complex in hospitals without a dedicated cardiovascular surgery service. Interhospital collaboration is essential in order to facilitate patient transfer under the best possible clinical conditions.
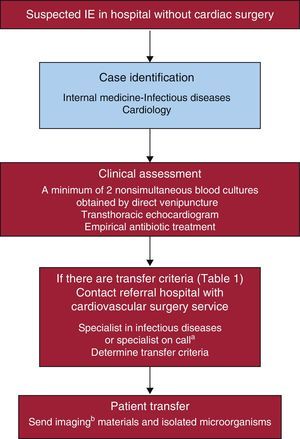
Overview of the ProcessWhen a patient has suspected IE in a secondary hospital, the diagnosis and treatment should be confirmed by contacting the tertiary hospital and sending imaging (echocardiography, computed tomography) and microbiological materials. It is important to clearly establish the criteria for patient transfer. Figure 3 shows the basic steps in this process.
It is recommended that physicians from the referring hospitals attend the weekly meeting, if possible, to present the cases and any imaging material. The MDT should share the endocarditis protocols with the physicians from the referring hospitals and should guarantee the continuing education of its physicians. The microbiology service of the referring hospital should store the strains of isolated microorganisms and send them to the reference laboratory for additional analysis.
It is recommended that indications for transferal are comprehensive. Table 1 shows the basic indications to consider a transfer to a referral center.
Criteria for the Transfer of Patients From Hospitals Without Cardiovascular Surgery
| 1. Patients with unstable hemodynamics (inotropic support, mechanical ventilation) |
| 2. Severe valvular regurgitation (clinical and echocardiographic criteria) |
| 3. Prosthetic endocarditis |
| 4. Intracardiac device endocarditis (pacemakers, defibrillators, resynchronizers) |
| 5. Extravalvular complications (abscesses, fistulas) |
| 6. Persistent sepsis (positive blood cultures > 7 days) |
| 7. Stroke |
| 8. Recurrent embolism |
| 9. Residual large vegetations (> 10 mm) |
| 10. Aggressive or difficult to treat microorganisms (eg, Staphylococcus aureus, Gram-negative bacilli, fungi, culture-negative endocarditis) |
The diagnosis and treatment of IE based on modified Duke criteria.12 The results of blood cultures should be obtained before antibiotics are administered. Echocardiographic examination should be performed within the first 48h. Patients with complicated IE on a native valve, artificial heart valve, or intracardiac prosthetic device should be admitted to the cardiology service and other patients with IE should be admitted to the infectious disease or internal medicine services. Patients with heart failure, septic shock, neurological disease, or conduction abnormalities should be admitted to intensive or intermediate care units. The treatment of IE is guided by good practice based on the clinical experience and judgment of the MDT and by the implementation of the approved recommendations of scientific societies.1
The severity of IE raises ethical problems in certain subgroups, such as patients with cancer, human immunodeficiency virus infection, or advanced liver cirrhosis. The MDT plays a key role in determining the benefits of surgical treatment in these subgroups of patients.
An institutional protocol ensures homogeneous treatment. The physician in charge of the patient must have the final responsibility, regardless of the opinion of the group. Medicolegal liability rests on the responsible physician.
CARE PROCESS OF THE PATIENT WITH INFECTIVE ENDOCARDITISIt is of particular importance to present the treatment options and their risks. The patient's family or equivalent must be informed about the situation and the treatment decisions made clear when the patient is unable to make them. Table 2 shows the process that should be followed in the care of patients with IE.
Actions to Be Taken During Admission and at Discharge of a Patient With Infective Endocarditis
| Before treatment |
| 1. Samples for blood cultures (3) by venipuncture before administration of antimicrobial agents |
| 2. Transthoracic echocardiography at the time of suspected infective endocarditis |
| 3. Transesophageal echocardiography for the definitive diagnosis and for the same indications as those described in Figure 2 |
| 4. Antibiogram and minimum inhibitory concentration (MIC) of the isolated microorganisms |
| 5. Placement of central venous line |
| 6. Electrocardiogram to detect rhythm or conduction abnormalities at baseline |
| 7. Laboratory analysis including serum biochemistry, C-reactive protein (or procalcitonin), complete blood count, coagulation profile, and urine sediment |
| 8. Simple chest X-ray in posteroanterior and lateral views if permitted by the patient's condition. If not, X-ray using a portable system in the hospital unit or special unit |
| During admission |
| 1. Clinical monitoring daily or according to clinical status |
| 2. Biochemical analysis every 3-4 days with special attention to kidney function and coagulation profile |
| 3. Transesophageal echocardiography if fever persists, if there are clinical changes, and before discharge. |
| 4. Monitor antibiotic concentrations |
| 5. Weekly medical-surgical meeting for case review and decision-making |
| 6. Immediate consultation with cardiovascular service if there are changes |
| 7. Assessment by outpatient parenteral antibiotic treatment team of patients with uncomplicated endocarditis |
| At discharge |
| 1. Clinical and laboratory control every week and at 1, 3, 6, and 12 months by the infectious disease service |
| 2. Control blood cultures at 48 h and 4 wk and 12 wk after completion of antibiotic treatment |
| 3. For patients who have not undergone surgery with moderate-to-severe residual valvular regurgitation, follow up by the cardiology service with echocardiography every 6 months to 12 months, depending on the severity of regurgitation |
| 4. For patients who have undergone surgery, annual follow-up by cardiac surgery service with echocardiography |
| 5. Recommendations for antibiotic prophylaxis for infective endocarditis |
Table 3 shows the list of signs and symptoms that should be monitored and tests and actions that must be performed during the treatment of a patient with IE.
Quality Control of the Multidisciplinary Working Group on Infectious Endocarditis
| Organizational aspects |
| 1. Provide response within 24 h to inquiries from other centers, including transfer requests made by telephone or via the internet |
| 2. Prospective collection of echocardiographic, microbiological, surgical, and clinical course data in a specialized database |
| 3. Weekly meetings with all members of the group |
| 4. Prevention of nosocomial endocarditis: provision of information and education to decrease catheter bacteremia and implantable cardiac device infections |
| Clinical aspects |
| 1. Echocardiography within 48 h of diagnosis of suspected infective endocarditis |
| 2. Embolic study within 72 h, especially in the case of fungal infectious endocarditis and staphylococcal endocarditis (Staphylococcus aureus, Staphylococcus lugdunensis) |
| 3. Regular review of the suitability, duration, and toxicity of empirical and definitive antibiotic regimens |
| Microbiological aspects |
| 1. A minimum of 2 nonsimultaneous blood cultures by direct venipuncture using different veins at the time of suspicion of IE. Control blood cultures at 3 days and 7 days |
| 2. Real-time communication from the group's microbiologist to the infectious disease specialist if growth is observed in blood cultures of typical pathogens of infective endocarditis (< 24 h) |
| 3. Staining, cultures, and molecular biology (16S and 18S) of all valvular vegetations, embolic material, and intracardiac devices of patients with suspected infective endocarditis |
| Surgical aspects |
| 1. In less than 24 h, discuss with the surgeon patients with indications for surgery |
| 2. Adhere to the time limits recommended in the guidelines for patients with urgent and emergency surgical indication |
| 3. Removal of all infected intracardiac devices and review and discuss patients in which this is not done |
| Scientific-academic issues |
| 1. Continuing education inside and outside the group |
| 2. Collaboration with local, national, and international study groups |
| 3. Communications to congresses; publications in scientific journals; participation in the development of local, national, and international clinical guidelines |
| General aspects |
| 1. Autopsy studies in more than 50% of inpatient deaths |
| 2. Clinical, microbiological, and echocardiographic follow-up for a minimum of 1 year in all patients |
Prevention is one of the most important aspects of IE.13 Clinical practice guidelines recommend antibiotic prophylaxis for patients with a previous episode of IE.1 Institutions and medical societies should disseminate information through informative brochures or via the Internet. Patient brochures about suspected IE and the need for dental checkup are prime educational materials for the general population. In order to prevent future episodes of IE, the patient and family should be provided with material on the disease, the procedures that can cause bacteremia, and the types of antibiotic prophylaxis.
The World Health Organization recommends education on hand-washing and compliance with protocols for zero bacteremia due to the risk of morbidity and mortality caused by venous catheter infections (including IE) in the hospital setting.
SURGICAL TREATMENTBetween 40% and 50% of patients with IE will need surgical treatment.2,11 In our hospital, the indications for surgical treatment in the acute phase are heart failure, conduction abnormalities, periannular complications, and persistent sepsis despite antibiotic treatment for at least 1 week.
The most important issue is the timing of the indication for surgery. Early surgery refers to when IE is diagnosed and treated at admission. Critically ill patients in a life-threatening situation need emergency treatment within 24h of diagnosis.
Stable patients should be treated within at least 1 week to control the infectious process, although the best moment for intervention has yet to be established for this group of patients. Using propensity-based matching adjustment for survival bias and instrumental variable analysis, Lalani et al14 found that patients with IE receiving antibiotic treatment should undergo surgery within 4 weeks of admission, which is the expected duration of parenteral therapy. Recently, Kang et al15 suggested intervention within 48h for patients with severe valvular regurgitation who have embolisms and vegetations (> 10mm) although improved survival was not demonstrated in this small sample of patients. Although the final results of the 2008 prospective randomized study ENDOVAL 1 (Rationale, Design and Methods for the Early Surgery in Infective Endocarditis Study, NCT00624091) remain pending, early results suggest that early intervention should be performed within 1-2 weeks of beginning antibiotic treatment.
Indications for surgery should be confirmed in the weekly MDT meeting. Urgent cases or life-threatening emergencies should be individually addressed rather than formally reviewed. Each patient is stratified according to preoperative surgical risk.
Overview of the Surgical ProcessOnce the indication for surgery has been established, the responsible surgeon must confirm the following steps:
- •
The patients have been included in the waiting list. Emergency cases have been dealt with as soon as possible.
- •
Informed consent has been obtained.
- •
The cardiac procedure, and extracardiac procedure if needed (splenectomy, etc), have been planned.
- •
Cardiac lesions have been confirmed by transesophageal echocardiography in the operating room before the start of the intervention.
- •
Potential intraoperative complications (standard checklist) have been predicted.
- •
Samples have been obtained for pathology and microbiology studies (stainings, cultures, and molecular studies).
- •
Confirm the orders have been stored in the computer system and that samples have arrived at the appropriate laboratories.
- •
Postoperative control has been performed in intensive and conventional care.
- •
Transthoracic echocardiography study has been confirmed before discharge.
The responsible physicians should follow up patients with IE who have undergone intervention. Patients should be followed up at 1, 3, and 12 months after intervention. Patients who have not undergone intervention should be followed up in medical outpatient consultations or at home after OPAT has been completed to assess possible recurrence and valvular dysfunction after the IE episode. Patients who have undergone intervention should be assessed in surgical outpatient consultations at 1, 3, and 12 months after intervention to follow-up cardiac and valvular function. Blood samples should be taken 48h after the completion of antibiotic treatment and at 1 and 3 months after discharge. The patient should be told that if they have fever they should go to hospital and should not take antibiotics to avoid false-negative results of blood cultures. The recurrence rate after the initial episode of IE is low (< 5%) and commonly occurs in the first 12 weeks. Infective endocarditis that develops after valve surgery is considered to be early IE if there is a new episode in the first 12 months and late IE if there is an episode after this period.
CONCLUSIONSInfective endocarditis is a rare disease that has an impact on the community due to its morbidity and consumption of resources. The key element in the treatment process is the MDT, which attempts to reach an early diagnosis, standardize treatment criteria, and optimize results. The MDT should review local cases of IE, facilitate the transfer of patients from hospitals that do not have a cardiovascular surgery unit, and follow up patients with IE after the initial episode. The MDT should undertake educational activities aimed at patients, familar environment, and professional colleagues with little experience with the disease. The MDT should also audit their activity according to the criteria specified in Table 3.
CONFLICTS OF INTERESTNone declared.
We would like to thank all our colleagues who over 30 years have helped us to improve the management of patients with IE at the Hospital Clínic de Barcelona. We would also like to thank Magda Heras, recently deceased, who always believed in the need for MDT to manage IE, facilitated the integration of cardiologists in the teams, and promoted their development. We will always be indebted to her. Finally, we thank Modesto Sánchez for designing the database and electronic medical records system for IE.
José M. Miró, Juan M. Pericás, Carlos Cervera, Cristina García de la María, Yolanda Armero, Asunción Moreno, José M. Gatell (Servicio de Enfermedades Infecciosas); Francesc Marco, Manel Almela, Jordi Vila (Servicio de Microbiología); Carlos A. Mestres, J. Carles Paré, Carlos Falces, Ramón Cartañá, Salvador Ninot, Manuel Azqueta, Marta Sitges, Magda Heras†, José L. Pomar (Servicio de Cardiología and Servicio de Cirugía Cardiovascular); José Ramírez (Servicio de Anatomía Patológica); Guillermina Fita, Irene Rovira (Servicio de Anestesiología, Reanimación y Terapéutica del Dolor); Mercè Brunet (Servicio de Toxicología); Dolors Soy (Servicio de Farmacia); David Fuster (Servicio de Medicina Nuclear) y Jaume Llopis (Departamento de Estadística, Universidad de Barcelona).