The implantation of bioresorbable scaffolds (BRS) is an emerging technique used in percutaneous coronary interventions. Their application has been extended to more complex lesions, although evidence is only available for simple lesions. The present study evaluated scaffold implantation in long lesions, focusing on overlapping scaffolds.
MethodsWe retrospectively analyzed all consecutive patients eligible for stenting with everolimus-eluting poly-L-lactic acid-based BRS with a minimum total scaffold length of 28mm, irrespective of the number of BRS used. The main target parameters were major adverse cardiac events, comprising cardiac death, any myocardial infarction, and target lesion revascularization, and target lesion failure, including cardiac death, target vessel myocardial infarction, and target lesion revascularization. A subgroup analysis included patients with overlapping BRS.
ResultsA total of 250 patients were included. The reason for angiography was stable coronary artery disease in 36.4% (91 of 250), an acute coronary syndrome in 61.6% (154 of 250), and other reasons in 2.0% (5 of 250). Procedural success was achieved in 97.8% (267 of 273) of the lesions. During follow-up, the 12-month rates of major adverse cardiac event, target lesion failure, and scaffold thrombosis were 8.5%, 6.6%, and 2.3%, respectively. Subgroup analysis of 239 patients showed that there were no statistically relevant differences between patients with and without overlapping scaffolds after a 12-month follow-up.
ConclusionsLong-segment stenting with a single scaffold or with multiple overlapping scaffolds is technically feasible with adequate mid-term outcomes. However, large-scale randomized studies are needed to provide further proof of concept.
Keywords
The implantation of bioresorbable scaffolds (BRS) is a promising new technique for the interventional treatment of coronary artery disease. Currently, the most widely investigated device is an everolimus-eluting, poly-L-lactic acid-based BRS (Absorb, Abbott Vascular; Santa Clara, California, United States).1 Recent randomized, controlled trials have demonstrated 1-year results that were equivalent to those obtained with drug-eluting metallic stents,2,3 and all-comers data show reasonable results in a broad spectrum of clinical settings and lesion types.4 Nevertheless, metallic drug-eluting stents (DES) are still the gold standard according to existing guidelines.5 Their outcome, however, especially when used in longer, more complex coronary stenoses, is relatively unfavorable since stent length is an independent predictor of in-stent restenosis as well as late and very late stent thrombosis. These adverse events are mostly related to incomplete arterial healing provoked by drug elution and a permanent metallic cage.6
Such adverse outcomes can potentially be avoided by implanting a BRS, which dissolves completely within 2-3 years.7 Procedural feasibility of BRS implantation has already been demonstrated in chronic total occlusions with adequate clinical short-term outcomes in small patient groups.8,9 Therefore, it is reasonable to assume that patients needing long-segment stenting could also benefit from the impermanent vessel support of a BRS. Certainly, there are some particularities associated with the strut thickness of 150μm and, in particular, the impact of overlapping BRS remains unclear. Thus, the aim of this study was to evaluate the mid-term outcome of patients with long-segment stenosis undergoing percutaneous coronary intervention with BRS implantation and to focus on the feasibility and outcomes of the use of overlapping BRS.
METHODSPatient PopulationFrom October 2012 to December 2015, all consecutive patients treated at the University of Giessen, Medizinische Klinik I, Department of Cardiology, Germany, within the scope of an all-comers registry and with a minimum total scaffold length of 28mm per vessel, irrespective of the number of BRS, were included in the study. To evaluate the potential impact of overlapping BRS on outcomes, the population with successful BRS implantation was divided post hoc into 2 subgroups to compare patients with overlapping BRS with those without overlap. We excluded patients treated with overlapping BRS at one site and not overlapping BRS at another site from this subgroup analysis.
The general inclusion criteria of this all-comers registry were any evidence of ischemia in a 12-lead electrocardiogram, elevated cardiac biomarkers or symptoms of angina pectoris, and angiographic eligibility for BRS implantation. Further details haven been published previously.10
Device and ProcedureThe circumferential and cross-linked struts of the Absorb BRS are made of poly-L-lactic acid with a thickness of 150μm. The Absorb BRS elutes a 1:1 mixture of poly-D, L-lactic acid and the antiproliferative drug everolimus. To ensure visualization, radiopaque markers are located at the tip of each end. In a porcine model, complete dissolution of the scaffold was observed after 2 years.7
The implantation of the BRS was performed according to standard clinical practice. Prior to the procedure, 70 U/kg body weight of unfractionated heparin was administered. For predilatation a noncompliant or, if required, a scoring balloon was used. Postdilatation was strongly recommended.
To guarantee a minimal overlap, the BRS were implanted in a marker-to-marker fashion: once the first BRS was implanted, the second BRS was positioned with its balloon marker directly over the marker of the first BRS. Thus, the radiopaque markers of both BRS were located directly next to each other. This technique leads to a minimal overlap zone of approximately 1mm.
Intravascular imaging included optical coherence tomography (ILUMIEN OPTIS, Dragonfly, St. Jude Medical, Inc.; St. Paul, Minnesota, United States) or intravascular ultrasound (Eagle Eye Gold, Volcano Corp.; San Diego, California, United States) and was used in certain cases for measurement of the lumen diameter and vessel characteristics according to the discretion of the implanting physician ().
Procedural success was defined as successful deployment of the BRS at the target lesion and an estimated residual stenosis equal to or less than 30% as visualized by angiography. The postprocedural antiplatelet regimen was administered according to existing guidelines.5
Baseline Evaluation and Follow-upBaseline evaluation contained documentation of the patients’ medical history, physical examination, 12-lead electrocardiogram, and blood laboratory examination. Follow-up was conducted either by office visits or by telephone interviews after 30 days, 6 months, 12 months, and then yearly.
All examinations were performed according to the Declaration of Helsinki. The patients received and signed a written consent form. This study was approved by the Ethics Board of the Justus Liebig University of Giessen, Giessen, Germany (AZ: 246/12).
Target ParametersMajor adverse cardiac events (MACE) consisted of cardiac death, myocardial infarction, or clinically driven percutaneous or surgical target lesion revascularization (TLR). The device-oriented composite endpoint of target lesion failure (TLF) consisted of cardiac death, target-vessel myocardial infarction, or TLR. Target vessel failure (TVF) included cardiac death, target vessel myocardial infarction, or clinically driven percutaneous or surgical target vessel revascularization. Scaffold thrombosis was defined according to Academic Research Consortium criteria.11 These endpoints were assessed after 1 year.
Statistical AnalysisContinuous variables are presented as mean±standard deviation or as median [interquartile range]. Categorical variables are presented as counts and percentages. To compare the 2 groups the Mann-Whitney-Wilcoxon U test was used for continuous variables, and the Fisher exact test or the chi-square test was applied for categorical variables. All tests were 2-tailed and a P-value<.05 was considered statistically significant. The Kaplan-Meier method was used to calculate event rates and the log rank test was used for comparisons.
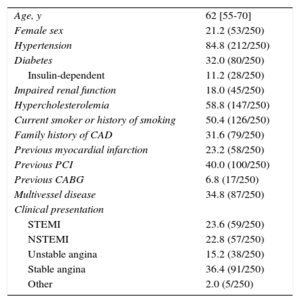
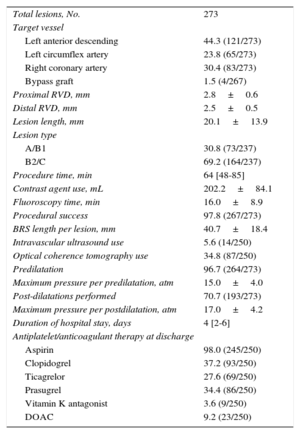
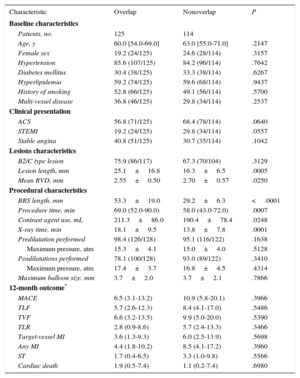
RESULTSBaseline and Procedural ResultsA total of 250 patients with a minimum total scaffold length of 28mm per vessel were found to be eligible for this investigation. Baseline characteristics are presented in Table 1. Catheterization was indicated due to an acute coronary syndrome in 61.6% (154 of 250), stable coronary artery disease in 36.4% (91 of 250), and other reasons in 2.0% (5 of 250). Radial access was used in 62.0% (155 of 250) and femoral access in 38.0% (95 of 250). Most lesions were localized at the left anterior descending coronary artery (44.3%), and 30.8% of the lesions were categorized as A/B1 and 69.2% as B2/C according to the American Heart Association/American College of Cardiology classification. An average of 1.8±0.9 BRS were implanted per lesion: 1 BRS was required in 44.0% (120 of 273) of the lesions, 2 BRS were required in 37.7 (103 of 273), and more than 3 BRS were implanted in 18.3% (50 of 273). In 6 lesions, BRS implantation was unsuccessful because it was not possible to cross the lesion with the BRS; thus, the lesion-based procedural success was 97.8% (267 of 273). Thrombus aspiration was performed in 43 ST-segment elevation myocardial infarction cases. In 18 cases (7.2%) an additional scoring balloon was used for predilatation; rotational atherectomy was not used. Intravascular imaging (optical coherence tomography or intravascular ultrasound) was used to guide BRS implantation in 40.4% of all patients. Further procedural details can be found in Table 2.
Baseline Characteristics
| Age, y | 62 [55-70] |
| Female sex | 21.2 (53/250) |
| Hypertension | 84.8 (212/250) |
| Diabetes | 32.0 (80/250) |
| Insulin-dependent | 11.2 (28/250) |
| Impaired renal function | 18.0 (45/250) |
| Hypercholesterolemia | 58.8 (147/250) |
| Current smoker or history of smoking | 50.4 (126/250) |
| Family history of CAD | 31.6 (79/250) |
| Previous myocardial infarction | 23.2 (58/250) |
| Previous PCI | 40.0 (100/250) |
| Previous CABG | 6.8 (17/250) |
| Multivessel disease | 34.8 (87/250) |
| Clinical presentation | |
| STEMI | 23.6 (59/250) |
| NSTEMI | 22.8 (57/250) |
| Unstable angina | 15.2 (38/250) |
| Stable angina | 36.4 (91/250) |
| Other | 2.0 (5/250) |
CABG, coronary artery bypass graft; CAD, coronary artery disease; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as median [interquartile range] or percentage (no/No.).
Procedural Details
| Total lesions, No. | 273 |
| Target vessel | |
| Left anterior descending | 44.3 (121/273) |
| Left circumflex artery | 23.8 (65/273) |
| Right coronary artery | 30.4 (83/273) |
| Bypass graft | 1.5 (4/267) |
| Proximal RVD, mm | 2.8±0.6 |
| Distal RVD, mm | 2.5±0.5 |
| Lesion length, mm | 20.1±13.9 |
| Lesion type | |
| A/B1 | 30.8 (73/237) |
| B2/C | 69.2 (164/237) |
| Procedure time, min | 64 [48-85] |
| Contrast agent use, mL | 202.2±84.1 |
| Fluoroscopy time, min | 16.0±8.9 |
| Procedural success | 97.8 (267/273) |
| BRS length per lesion, mm | 40.7±18.4 |
| Intravascular ultrasound use | 5.6 (14/250) |
| Optical coherence tomography use | 34.8 (87/250) |
| Predilatation | 96.7 (264/273) |
| Maximum pressure per predilatation, atm | 15.0±4.0 |
| Post-dilatations performed | 70.7 (193/273) |
| Maximum pressure per postdilatation, atm | 17.0±4.2 |
| Duration of hospital stay, days | 4 [2-6] |
| Antiplatelet/anticoagulant therapy at discharge | |
| Aspirin | 98.0 (245/250) |
| Clopidogrel | 37.2 (93/250) |
| Ticagrelor | 27.6 (69/250) |
| Prasugrel | 34.4 (86/250) |
| Vitamin K antagonist | 3.6 (9/250) |
| DOAC | 9.2 (23/250) |
BRS, bioresorbable scaffold; DOAC, direct oral anticoagulant agent; RVD, reference vessel diameter.
Data are expressed as mean±standard deviation, median [interquartile range] or percentage (no./No.).
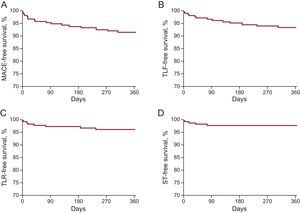
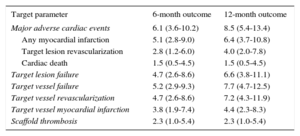
The median length of follow-up was 362 [169.3-708.8] days; 62.1% of the patients (149 of 240) completed at least the 12 months’ follow-up and 2.4% (6 of 250) were lost to follow-up. An overview of clinical outcomes is presented in Table 3. Event rates after 1 year were 8.5% for MACE, 6.6 for TLF, 4.0% for TLR, 2.3% for scaffold thrombosis, and 1.5% for cardiac deaths (Figure 1). The 5 cases of scaffold thrombosis occurred 24hours and 4, 17, 38 and 77 days after BRS implantation, with 2 of these patients having discontinued their antiplatelet therapy. All incidences of scaffold thrombosis were classified as definite according to Academic Research Consortium criteria.
Clinical Outcome
| Target parameter | 6-month outcome | 12-month outcome |
|---|---|---|
| Major adverse cardiac events | 6.1 (3.6-10.2) | 8.5 (5.4-13.4) |
| Any myocardial infarction | 5.1 (2.8-9.0) | 6.4 (3.7-10.8) |
| Target lesion revascularization | 2.8 (1.2-6.0) | 4.0 (2.0-7.8) |
| Cardiac death | 1.5 (0.5-4.5) | 1.5 (0.5-4.5) |
| Target lesion failure | 4.7 (2.6-8.6) | 6.6 (3.8-11.1) |
| Target vessel failure | 5.2 (2.9-9.3) | 7.7 (4.7-12.5) |
| Target vessel revascularization | 4.7 (2.6-8.6) | 7.2 (4.3-11.9) |
| Target vessel myocardial infarction | 3.8 (1.9-7.4) | 4.4 (2.3-8.3) |
| Scaffold thrombosis | 2.3 (1.0-5.4) | 2.3 (1.0-5.4) |
Data are expressed as percentages by Kaplan-Meier estimates (95% confidence interval).
Clinical outcomes at 12 months among patients undergoing long-segment stenting with BRS. A: MACE, comprising cardiac death, myocardial infarction or TLR. B: TLF, comprising cardiac death, target-vessel myocardial infarction or TLR. C: TLR. D: ST. BRS, bioresorbable scaffold; MACE, major adverse cardiac events; ST, scaffold thrombosis; TLF, target lesion failure; TLR, target lesion revascularization.
Of all patients in the cohort, 239 met the criteria for subgroup analysis. Of these, 125 were treated with overlapping BRS and 114 were treated without overlapping BRS (Table 4). No differences were found between groups in baseline characteristics or clinical presentation. Femoral access was used similarly in both groups (40.8% vs 34.2%; P=.2937). General procedural parameters including procedure time, fluoroscopy time and contrast use were higher in the overlap group, but pre- and postdilatation were applied in a similar proportion (Table 4).
Subgroup Analysis
| Characteristic | Overlap | Nonoverlap | P |
|---|---|---|---|
| Baseline characteristics | |||
| Patients, no. | 125 | 114 | |
| Age, y | 60.0 [54.0-69.0] | 63.0 [55.0-71.0] | .2147 |
| Female sex | 19.2 (24/125) | 24.6 (28/114) | .3157 |
| Hypertension | 85.6 (107/125) | 84.2 (96/114) | .7642 |
| Diabetes mellitus | 30.4 (38/125) | 33.3 (38/114) | .6267 |
| Hyperlipidemia | 59.2 (74/125) | 59.6 (68/114) | .9437 |
| History of smoking | 52.8 (66/125) | 49.1 (56/114) | .5700 |
| Multi-vessel disease | 36.8 (46/125) | 29.8 (34/114) | .2537 |
| Clinical presentation | |||
| ACS | 56.8 (71/125) | 68.4 (78/114) | .0640 |
| STEMI | 19.2 (24/125) | 29.8 (34/114) | .0557 |
| Stable angina | 40.8 (51/125) | 30.7 (35/114) | .1042 |
| Lesions characteristics | |||
| B2/C type lesion | 75.9 (86/117) | 67.3 (70/104) | .3129 |
| Lesion length, mm | 25.1±16.8 | 16.3±6.5 | .0005 |
| Mean RVD, mm | 2.55±0.50 | 2.70±0.57 | .0250 |
| Procedural characteristics | |||
| BRS length, mm | 53.3±19.0 | 29.2±6.3 | <.0001 |
| Procedure time, min | 69.0 (52.0-90.0) | 58.0 (43.0-72.0) | .0007 |
| Contrast agent use, mL | 211.3±86.0 | 190.4±78.4 | .0248 |
| X-ray time, min | 18.1±9.5 | 13.8±7.8 | .0001 |
| Predilatation performed | 98.4 (126/128) | 95.1 (116/122) | .1638 |
| Maximum pressure, atm | 15.3±4.1 | 15.0±4.0 | .5128 |
| Posdilatations performed | 78.1 (100/128) | 93.0 (89/122) | .3410 |
| Maximum pressure, atm | 17.4±3.7 | 16.8±4.5 | .4314 |
| Maximum balloon size, mm | 3.7±2.0 | 3.7±2.1 | .7866 |
| 12-month outcome* | |||
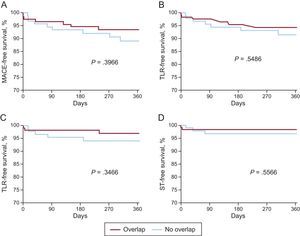
| MACE | 6.5 (3.1-13.2) | 10.9 (5.8-20.1) | .3966 |
| TLF | 5.7 (2.6-12.3) | 8.4 (4.1-17.0) | .5486 |
| TVF | 6.6 (3.2-13.5) | 9.9 (5.0-20.0) | .5390 |
| TLR | 2.8 (0.9-8.6) | 5.7 (2.4-13.3) | .3466 |
| Target-vessel MI | 3.6 (1.3-9.3) | 6.0 (2.5-13.9) | .5698 |
| Any MI | 4.4 (1.8-10.2) | 8.5 (4.1-17.2) | .3960 |
| ST | 1.7 (0.4-6.5) | 3.3 (1.0-9.8) | .5566 |
| Cardiac death | 1.9 (0.5-7.4) | 1.1 (0.2-7.4) | .6980 |
ACS, acute coronary syndrome; BRS, bioresorbable scaffold; MACE, major adverse cardiac event; MI, myocardial infarction; RVD, reference vessel diameter; STEMI, ST-segment elevation myocardial infarction; TLF, target lesion failure; TLR, target lesion revascularization; TVF, target vessel failure; ST, definite scaffold thrombosis according to Academic Research Consortium criteria.
Unless otherwise indicated, data are expressed as mean±standard deviation, median [interquartile range] or percentage (no/No.).
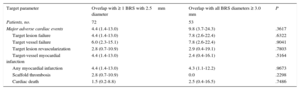
No differences were detected in the 12-month outcome during follow-up (Figure 2). When we compared patients in the total cohort with and without MACE, overlapping implantation of BRS was performed in the same proportion (47.1% [8 of 17] vs 54.9% [128 of 233]; P=.5290). In a Cox-regression analysis adjusted for age, sex and clinical presentation, overlap was not a predictor of MACE (P=.901; hazard ratio=1.056; 95% confidence interval, 0.446-2.499). Comparison of the outcome of patients with at least 1 overlapping BRS of 2.5mm in diameter (n=72) with those having overlapping BRS only larger than 2.5mm (n=53) revealed that the rates of MACE, TVF, TLR, and cardiac death were similar (Table 5).
Comparison of the 12-month outcomes of patients with and without overlapping BRS. A: MACE, comprising cardiac death, myocardial infarction or TLR. B: TLF, comprising cardiac death, target-vessel myocardial infarction or TLR. C: TLR. D: ST. BRS, bioresorbable scaffold; MACE, major adverse cardiac events; ST, scaffold thrombosis; TLF, target lesion failure; TLR, target lesion revascularization.
Comparison of Clinical Outcomes After 12 Months Between Patients Treated With and Without 2.5mm Overlapping Bioresorbable Scaffold
| Target parameter | Overlap with ≥ 1 BRS with 2.5mm diameter | Overlap with all BRS diameters ≥ 3.0 mm | P |
|---|---|---|---|
| Patients, no. | 72 | 53 | |
| Major adverse cardiac events | 4.4 (1.4-13.0) | 9.8 (3.7-24.3) | .3617 |
| Target lesion failure | 4.4 (1.4-13.0) | 7.8 (2.6-22.4) | .6322 |
| Target vessel failure | 6.0 (2.3-15.1) | 7.8 (2.6-22.4) | .9041 |
| Target lesion revascularization | 2.8 (0.7-10.9) | 2.9 (0.4-19.1) | .7803 |
| Target-vessel myocardial infarction | 4.4 (1.4-13.0) | 2.4 (0.4-16.1) | .5164 |
| Any myocardial infarction | 4.4 (1.4-13.0) | 4.3 (1.1-12.2) | .9673 |
| Scaffold thrombosis | 2.8 (0.7-10.9) | 0.0 | .2298 |
| Cardiac death | 1.5 (0.2-8.8) | 2.5 (0.4-16.5) | .7486 |
BRS, bioresorbable scaffold.
Data are expressed as percentages by Kaplan-Meier estimates (95% confidence interval).
The present study investigated a subpopulation of an all-comers registry of patients treated with a minimum BRS length of 28mm. Experience in long-segment stenting with BRS and in overlapping BRS is very limited, and this analysis comprises the largest evaluation of BRS use in long lesions reported thus far. The major findings are the following: a) long-segment stenting with BRS is technically feasible with adequate reasonable mid-term outcomes. The rates of MACE and TLF were low, and the rate of stent thrombosis was driven by patient with premature discontinuation of dual antiplatelet therapy; b) the implantation of overlapping scaffolds can be performed successfully with similar outcomes compared with patients without BRS overlap, and c) overlaps including at least one 2.5-mm BRS seem to be as safe as overlaps with the use of BRS larger than 2.5mm in terms of the 12-month clinical results.
Lesion length as well as stent length and lesion complexity are well known independent predictors of in-stent restenosis among all types of metallic stents.12–14 Furthermore, one of the anticipated major drawbacks of conventional DES is the occurrence of late stent thrombosis, caused by delayed arterial healing due to drug elution. The permanent metallic implant also results in chronic inflammatory reactions and deterioration of endothelial function, leading to increased thrombogenicity. Persistent malapposition and stent overlap even raise the risk of late stent thrombosis.6,15 In particular, overlapping sites have shown greater evidence of chronic inflammation and delayed healing and consequently a further increase thrombotic risk and worsened clinical outcomes.16,17 These disadvantages may be overcome with BRS, since there is no permanent implant because they dissolve within 24 to 36 months. In addition, BRS allow mid-term scaffolding of the previously stenosed vessel site and are associated with positive side effects, most notably a late lumen enlargement, recovery of vasomotion, less plaque progression, and a reduction of angina.1,2,18–20
There are, however, mechanical drawbacks related to the strut thickness of 150μm and the resulting thicker crossing profile, which limit BRS use, especially in the presence of severe calcification and tortuosity. Long lesions also appear to be suboptimal for BRS use, especially when overlapping BRS are required, resulting in a total strut thickness of 300μm at the overlapping site and a total of 600μm when considering the diameter of the vessel. Furthermore, a previous study of DES showed that thicker stent struts are unfavorable in complex lesions.21 Most of the existing experience with BRS has been acquired from studies investigating simple lesions in patients with stable coronary artery disease.22
The present analysis evaluated especially long-segment stenting with BRS, as evidence regarding these types of lesions is very rare. The results of small studies that have dealt with chronic total occlusion treated with BRS have been published previously.8,9 In these 2 investigations, the scaffold length per lesion was 64.8±24.2 and 52.5±22.9mm and TLR rates were 4.3% and 0.0%, respectively. These principal findings were similar to the results presented here, showing a TLR rate of 4.0%. Moreover, large-scale, nonrandomized investigations with similar baseline characteristics have been carried out with 1189 and 512 patients with 51.2% and 41% B2/C lesions and a mean lesion length of 19.4mm and 11.9mm, respectively.4,23 After 6 months, TLR rates were 2.5% and 0.6%, scaffold thrombosis occurred in 2.1% and 0.6%, and TVF was noted in 4.9% and 3.3%, respectively.4,23 These findings were in part similar to the outcomes presented here, although the proportion of B2/C lesions (69.2%) in our study was considerably higher.
However, previous studies did not focus on overlapping stents and also included nonoverlapping scaffolds or patients treated with only 1 BRS. Only a few cases of successful BRS overlap have been described.24,25 In a porcine model, overlapping BRS were evaluated by optical coherence tomography after 28 and 90 days and were compared with DES.26 The major findings were that overlapping BRS showed delayed strut coverage after 28 days, mostly related to overlapping BRS struts, which was resolved after 90 days. Furthermore, an increased neointimal response was observed when BRS were used, but this did not result in a significantly greater volume obstruction compared with DES. These results were mostly attributed to the greater strut thickness of the BRS.26 Of note, no significant differences were observed regarding the clinical outcomes after 1 year in a matched analysis of 70 patients with overlapping BRS compared with 70 patients with overlapping DES.25
A close look at patients with overlap suggests that those treated with at least 1 BRS with a diameter of 2.5mm had similar results. However, the total scaffold thickness is 600μm at the overlapping site and thus it is difficult to achieve a relevant lumen gain in smaller vessels. Furthermore, neointimal growth might lead to a more distinct risk of BRS failure at the overlapping site since the margin for ischemia is lower, especially in small vessels. Therefore, implantation of overlapping BRS should be reserved for highly selected patients.
Taking into account all patients in this cohort treated with overlapping BRS, the present findings are consistent with those obtained for DES: after 30 days the TLR rate varied between 0.0% and 3.8% and increased up to 8.2% after 1 year, depending on the type of DES used.27 Furthermore, the stent thrombosis rate was between 0.0% and 3.8% after 30 days, which did not change for up to 1 year. In the present study, the incidence of scaffold thrombosis was mostly driven by events during the early phase after implantation.
LimitationsThese real-world data were gathered in a nonrandomized fashion and there were no routinely performed angiographic follow-up examinations. Although the follow-up completion rate was high, some patients were lost to follow-up and the possibility that these patients experienced adverse events cannot be definitely ruled out. Post hoc subgroup analysis was performed without matching. Baseline characteristics, clinical presentation, and lesion classification, however, did not vary statistically significantly, and differences in procedural characteristics were expected. Due to the small number of patients, all results should to be interpreted with caution, especially regarding the stent thrombosis rate in the subgroup analysis of patients with overlaps including a 2.5mm BRS or larger BRS. However, a post hoc power calculation showed a power of 78.8% to detect differences in MACE. The number of patients is relatively low, and thus the statistical significance might be under- or overpowered.
CONCLUSIONsIn a real-world scenario with an all-comers group of patients, stenting with a total BRS length of 28mm or more as well as implantation of overlapping BRS can be performed with good clinical mid-term outcomes. Bioresorbable scaffolds would appear to be well suited for use with long lesions due to a number of advantages over conventional DES. Mechanical drawbacks must be considered, however, and implantation of overlapping BRS should be performed cautiously. Larger, randomized, controlled trials and long-term data will be required to test the concept and assumptions regarding the advantages and disadvantages of BRS implantation.
Conflicts of interestA. Elsässer, H. Möllmann, C. Hamm, and H. Nef received honoraria for lectures from Abbott Vascular. H. Nef also received a research grant from Abbott Vascular. J. Wiebe received travel expenses from Abbott Vascular.
- -
Bioresorbable scaffolds demonstrate reasonable clinical outcomes compared with standard metallic DES. However, most of the available data were gathered from studies investigating predominately simple lesions.
- -
Experience with BRS implantation in more complex anatomical settings is sparse. For the treatment of long lesions with BRS in particular, some particularities need to be considered, eg, when an overlap of 2 BRS with a strut thickness of 150μm each is required.
- -
Long-segment stenting with BRS shows a high technical success rate and the mid-term outcome is satisfactory. Furthermore, outcomes are similar in patients receiving overlapping scaffolds and those without BRS overlap. Although there are some concerns about overlaps including 2.5mm BRS, the clinical results seem to be similar to overlaps with the use of BRS larger than 2.5mm. However, these findings require confirmation in further large-scale studies.
The authors would like to thank Elizabeth Martinson, PhD, for editorial assistance.