Noninvasive ventilation (NIV) has been shown to reduce the rate of endotracheal intubation and mortality in patients with acute heart failure (AHF). However, patients with AHF secondary to acute coronary syndrome/acute myocardial infarction (ACS-AMI) have been excluded from many clinical trials. The purpose of this study was to compare the effectiveness of NIV between patients with AHF triggered by ACS-AMI and by other etiologies.
MethodsProspective cohort study of all patients with AHF treated with NIV admitted to the intensive care unit for a period of 20 years. Patients were divided according to whether they had ACS-AMI as the cause of the AHF episode. NIV failure was defined as the need for endotracheal intubation or death.
ResultsA total of 1009 patients were analyzed, 403 (40%) showed ACS-AMI and 606 (60%) other etiologies. NIV failure occurred in 61 (15.1%) in the ACS-AMI group and in 64 (10.6%) in the other group (P=.031), without differences in in-hospital mortality (16.6% and 14.9%, respectively; P=.478).
ConclusionsThe presence of ACS-AMI as the triggering cause of AHF did not influence patients with acute respiratory failure requiring noninvasive respiratory support.
Keywords
Respiratory involvement is very common in patients with acute heart failure (AHF).1 The increase in hydrostatic pressure in the pulmonary vasculature, secondary to the increased pressure in the left atrium, causes an increase in fluid accumulation in the pleural and alveolar spaces.2 This, in turn, leads to a decrease in pulmonary compliance, increased respiratory work and the development of acute hypoxemia and, frequently, hypercapnia.3
Among AHF triggers, acute coronary syndrome-acute myocardial infarction (ACS-AMI) is one of the most frequent, affecting up to one third of patients, and even more in patients with newly initiated or “de novo” AHF.4 Heart failure complicating acute myocardial infarction (AMI) is frequent,5 being one of the main predictors of death.6,7
In recent decades, there have been important changes in the incidence and outcomes of patients with heart failure due to the introduction of new drugs, the creation of special diagnostic and follow-up units, and technological advances related to myocardial revascularization techniques, cardiac surgery, extracorporeal ventricular assistance, and heart transplant.1 However, the treatment of AHF in the emergency room or critical care unit (ICU) has remained unchanged. The application of noninvasive ventilation (NIV) has been extensively studied in AHF.8 In a recent meta-analysis,9 the application of NIV reduced the need for intubation and in-hospital mortality compared with conventional oxygen therapy. However, some clinical trials have excluded patients with ACS-AMI. Consequently, the recommendation of the use of NIV in patients with acute cardiogenic lung edema cannot be applied to patients with ACS.10 In addition, the presence of AHF triggered by ACS-AMI has been related to a worse prognosis, although the results of studies are contradictory.11–18
We hypothesized that patients with AHF due to ACS-AMI treated with NIV might have a worse prognosis. The main objective of this study was to analyze NIV failure, defined as the need for intubation or death in the ICU, in patients with AHF with and without ACS-AMI. The secondary objectives were to compare hospital and yearly mortality between those 2 groups.
METHODSPatientsIn this cohort study, we prospectively analyzed all patients who were consecutively admitted with a diagnosis of AHF and received treatment with NIV in an 18-bed ICU of a university hospital. The analyzed patients were those admitted between January 1997 and December 2017. The study was approved by the ethics committee of the institution.
Patients were included when the following criteria were met: a) clinical criteria: respiratory distress: presence of respiratory rate greater than 30 breaths per minute, or activity of the accessory respiratory muscles, or abdominal paradoxical breathing; physical examination: presence of bilateral crackles accompanied or not by wheezing, or auscultation of a third cardiac sound; ortopnea; severe acute or chronic on acute respiratory failure: presence of PaO2/FiO2 less than 250mmHg and/or respiratory acidosis (arterial pH less than 7.35 with PaCO2 greater than 45mmHg); b) confirmation of pulmonary congestion (at least 2 of the following): signs of pulmonary congestion on a chest imaging test; 3 or more B lines on the thoracic ultrasound in 2 thoracic areas in each hemithorax; elevation of pulmonary capillary pressure (> 18mmHg) shown by right heart catheterization; increase in total lung water by pulse contour analysis and thermodilution; echocardiographic signs of elevation of ventricular filling pressures; significant elevation of N-terminal pro-B-type natriuretic peptide above normal levels according to the patient's age. Exclusion criteria were those usually considered.19
The episode of AHF was classified according to its etiology as secondary to ACS-AMI or due to another cause. The diagnosis of ACS-AMI was established according to the current criteria at the time of admission.20–22 During the first years of the study, patients were classified as having non-Q wave myocardial infarction and Q wave myocardial infarction. These patients were subsequently reclassified as non–ST-segment elevation ACS and ST-segment elevation myocardial infarction, or indeterminate (presence of pacemaker or left branch full block). All electrocardiograms were evaluated by 2 intensive care physicians with extensive experience in coronary patients and cases of doubt being decided by the opinion of a third intensivist. Diagnosis of AMI required corroboration from the cardiologist who treated the patient in the admissions ward.
“De novo” AHF was defined as the absence of a previous history of heart failure.23
AHF patients’ treatment was carried out according to current guidelines at the time of hospital admission. The center where the study was carried out did not have a cardiac catheterization laboratory, and coronary studies were performed at the referral center 10km away, the transfer being carried out by medicalized ambulance with availability of NIV and medical and nursing personnel previously trained for this therapy. Between 1997 and 1999, in patients who needed urgent coronary angiography, that intervention was performed in the morning of the admission day (08:00-15:00) or on the next day if they were admitted after 15:00. From 2000, the availability of urgent coronary angiography was extended to 24hours a day, every day of the year. Coronary angiography studies were defined as immediate or primary when performed at the time of STEMI diagnosis, or delayed if performed after at least 24hours of diagnosis. The treatment of AHF was mainly based on the use of vasodilators and loop diuretics, both in continuous perfusion. All patients received intravenous morphine, to decrease anxiety and dyspnea, and improve adaptation to NIV, except for those with an impaired level of consciousness. The use of dobutamine or levosimendan was restricted to patients not responding to the initial treatment and showing a depressed left ventricular ejection fraction (LVEF) on cardiac ultrasound. All patients underwent bedside echocardiography in the first 24hours of stay in the ICU.
Noninvasive ventilation protocolNIV was performed using specific noninvasive ventilators (BiPAP ST-D ventilator and VISION ventilator from Respironic Inc, USA; and V60 ventilator from Phillips Respironic, USA), using bilevel positive airway pressure, as previously published.19 The interface chosen in the first place was facial mask, with the nasal or the total facial mask being used when the patient showed interface-related complications. NIV was initiated with a positive inspiratory airway pressure of at least 12 cmH2O, with increments of 2 to 3 cmH2O if tolerated by the patient and according to clinical response, not exceeding 25 cmH2O. Positive expiratory pressure was initially 5 cmH2O, increasing by 1 to 2 cmH2O to improve hypoxemia or patient comfort. If the continuous positive airway pressure (CPAP) mode was used, due to intolerance to the bilevel mode, a continuous positive pressure of 7 cmH2O was used, with the possibility of rising up to 15 cmH2O. The inspired oxygen fraction (FiO2) administered was that necessary to reach an SpO2 of 92%. Since 2012, high-flow oxygen therapy through nasal cannula has been used in patients not tolerating NIV or to facilitate weaning from it, alternating the 2 respiratory therapy devices.
Noninvasive ventilation effectivenessNIV failure was assumed when the patient showed a deterioration of gas exchange parameters, or worsening of signs and symptoms of respiratory failure, despite optimization of NIV settings, leading to intubation or death during the ICU stay or during the first 24hours in the ward after discharge from the ICU, without recovery of the respiratory process motivating the need for noninvasive ventilatory support. In case of NIV failure, the patient was intubated and connected to invasive mechanical ventilation, according to previously published criteria.19
MeasurementsAt the beginning of NIV treatment, demographic, clinical and laboratory variables were collected. As in other studies, to quantify the patients’ comorbidity, the Charlson comorbidity index was calculated, the degree of multiorgan dysfunction was assessed through the Sequential Organ index Failure Assessment (SOFA), calculated daily, and disease severity was obtained by calculating the Simplified Acute Physiology Score II (SAPS II) index.14,19 Anemia was defined as the presence of hemoglobin less than 12g/L in women and less than 13 in men. The location of the ACS-AMI was classified as anterior, inferior or indeterminate, by means of the alterations shown in the electrocardiogram and echocardiography. ACS-AMI of lateral location were included in the inferior or anterior group, depending on the involvement of these cardiac regions. The determinations of cardiac injury markers changed over time; the level of creatine phosphokinase fraction Mb was used for the first 5 years of the study and after that troponin I. The patients were monitored up to 1 year after admission, to analyze mortality and eventual readmissions.
Statistical analysisQualitative variables are shown as absolute and relative frequencies, and comparisons between them were made using the Pearson chi-square test or Fisher test. Quantitative variables are expressed as mean±standard deviation or median [interquartile range], and comparisons between independent groups were made by Student t test or Mann-Whitney U test if the variable did not follow normal distribution. The Student t test was used for related data to analyze the quantitative variables measured before and after NIV initiation. The association measures analyzed were odds ratios (OR) with their 95% confidence intervals (95%CI). The changes per year was assessed through survival analysis and comparison between groups by Cox analysis. A paired propensity analysis was performed, “nearest neighbor” mode, with a 1:1 ratio, using the matching variables: age, sex, SAPS II, initial SOFA, Charlson index, and the presence of nonintubation order. All analyses were performed by bilateral contrast, and a P value less ≤ .05 was considered significant. The analysis was performed using the SPSS 25.0 program (IBM, Armonk, NY, United States) and R version 3.4.0 (Copyright 2017 The R Foundation for Statistical Computing Platform).
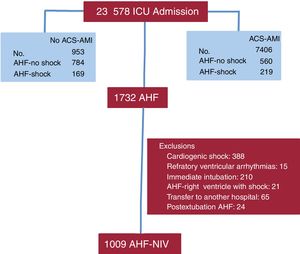
RESULTSDuring the study period, 7406 ACS-AMI patients were admitted to the ICU, of which 779 had AHF. In addition, there were 953 admissions for AHF without ACS-AMI. A total of 1732 patients with AHF were evaluated; 723 were excluded for different reasons and finally we analyzed 1009 AHF patients (figure 1).
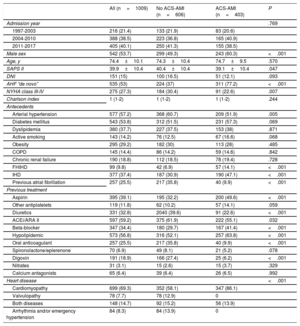
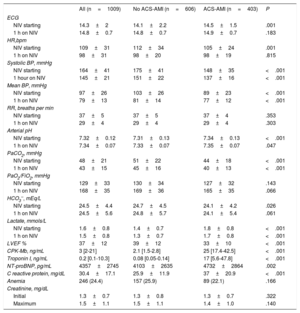
The main characteristics of the patients are shown in table 1. There was a slight predominance of male sex (53.7%), with a larger number of men in the ACS-AMI group. However, neither age (mean of 74 years) nor comorbidity index differed between the 2 groups. SAPS II score was slightly higher in the group without AMI. In patients with ACS-AMI, “de novo” AHF predominated. Among antecedents, hypertension and previous atrial fibrillation were more frequent in the group without infarction, while family history and previous ischemic heart disease were more common in the ACS-AMI group.
Demographic and clinical variables, antecedents and previous treatment
| All (n=1009) | No ACS-AMI (n=606) | ACS-AMI (n=403) | P | |
|---|---|---|---|---|
| Admission year | .769 | |||
| 1997-2003 | 216 (21.4) | 133 (21.9) | 83 (20.6) | |
| 2004-2010 | 388 (38.5) | 223 (36.8) | 165 (40.9) | |
| 2011-2017 | 405 (40.1) | 250 (41.3) | 155 (38.5) | |
| Male sex | 542 (53.7) | 299 (49.3) | 243 (60.3) | <.001 |
| Age, y | 74.4±10.1 | 74.3±10.4 | 74.7±9.5 | .570 |
| SAPS II | 39.9±10.4 | 40.4±10.4 | 39.1±10.4 | .047 |
| DNI | 151 (15) | 100 (16.5) | 51 (12.1) | .093 |
| AHF “de novo” | 535 (53) | 224 (37) | 311 (77.2) | <.001 |
| NYHA class III-IV | 275 (27.3) | 184 (30.4) | 91 (22.6) | .007 |
| Charlson index | 1 (1-2) | 1 (1-2) | 1 (1-2) | .244 |
| Antecedents | ||||
| Arterial hypertension | 577 (57.2) | 368 (60.7) | 209 (51.9) | .005 |
| Diabetes mellitus | 543 (53.8) | 312 (51.5) | 231 (57.3) | .069 |
| Dyslipidemia | 380 (37.7) | 227 (37.5) | 153 (38) | .871 |
| Active smoking | 143 (14.2) | 76 (12.5) | 67 (16.6) | .068 |
| Obesity | 295 (29.2) | 182 (30) | 113 (28) | .485 |
| COPD | 145 (14.4) | 86 (14.2) | 59 (14.6) | .842 |
| Chronic renal failure | 190 (18.8) | 112 (18.5) | 78 (19.4) | .728 |
| FHIHD | 99 (9.8) | 42 (6.9) | 57 (14.1) | <.001 |
| IHD | 377 (37.4) | 187 (30.9) | 190 (47.1) | <.001 |
| Previous atrial fibrillation | 257 (25.5) | 217 (35.8) | 40 (9.9) | <.001 |
| Previous treatment | ||||
| Aspirin | 395 (39.1) | 195 (32.2) | 200 (49.6) | <.001 |
| Other antiplatelets | 119 (11.8) | 62 (10.2) | 57 (14.1) | .059 |
| Diuretics | 331 (32.8) | 2040 (39.6) | 91 (22.6) | <.001 |
| ACEi/ARA II | 597 (59.2) | 375 (61.9) | 222 (55.1) | .032 |
| Beta-blocker | 347 (34.4) | 180 (29.7) | 167 (41.4) | <.001 |
| Hypolipidemic | 573 (56.8) | 316 (52.1) | 257 (63.8) | <.001 |
| Oral anticoagulant | 257 (25.5) | 217 (35.8) | 40 (9.9) | <.001 |
| Spironolactone/eplerenone | 70 (6.9) | 49 (8.1) | 21 (5.2) | .078 |
| Digoxin | 191 (18.9) | 166 (27.4) | 25 (6.2) | <.001 |
| Nitrates | 31 (3.1) | 15 (2.6) | 15 (3.7) | .329 |
| Calcium antagonists | 65 (6.4) | 39 (6.4) | 26 (6.5) | .992 |
| Heart disease | <.001 | |||
| Cardiomyopathy | 699 (69.3) | 352 (58.1) | 347 (86.1) | |
| Valvulopathy | 78 (7.7) | 78 (12.9) | 0 | |
| Both diseases | 148 (14.7) | 92 (15.2) | 56 (13.9) | |
| Arrhythmia and/or emergency hypertension | 84 (8.3) | 84 (13.9) | 0 | |
ACEI, angiotensin converting enzyme inhibitor; ACS-AMI, acute coronary syndrome-acute myocardial infarction; AHF, acute heart failure; ARA II, antagonist of the angiotensin receptors 2; COPD, chronic obstructive pulmonary disease; DNI, do not intubate order; FHIHD, family history of ischemic heart disease; IHD, ischemic heart disease; NYHA, New York Heart Association; SAPS, Simplified Acute Physiology Score.
The data are expressed as No. (%) or mean±standard deviation.
In the ACS-AMI group, 186 patients (46.2%) had STEMI, 207 (51.4%) non–ST-elevation acute myocardial infarction and 10 (2.5%) were undetermined. The most common infarction location was anterior (320 cases, 79.4%), followed by inferior (75 cases, 18.6%) and 8 cases (2%) were of undetermined location. Coronary angiography was performed in 369 patients (91.6%); in 133 (36%) angiography was immediate. Another 20 (13.1%) patients received systemic fibrinolysis. The vessel responsible for ACS-AMI was the left main artery in 43 cases (11.7%), the anterior descending artery in 214 (58%), the circumflex artery in 49 (13.3%), and the right coronary artery in 63 cases (17.1%).
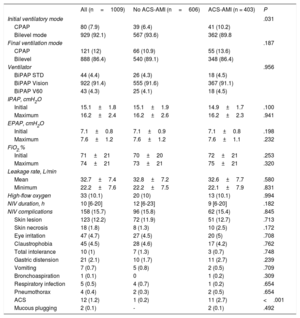
Physiological measurementsRespiratory, hemodynamic and neurological parameters before and after 1 hour of NIV are presented in table 2. The initial values showed higher blood pressure and heart rate in the group without ACS-AMI with lower pH and higher PaCO2, as well as a worse neurological status. Bicarbonate and arterial lactate values also showed differences between the 2 groups, with a lower level (for bicarbonate) and a higher level (for lactate) in the ACS-AMI group. Although these differences were statistically significant, they were not clinically relevant. In both groups, all variables improved significantly at the time of ventilatory therapy (all P values <.001), except for arterial pH and serum bicarbonate in the ACS-AIM group and serum bicarbonate in the non–ACS-AMI group. The mean LVEF of the patients analyzed showed a greater compromise in the ACS-AMI group.
Neurologic, hemodynamic, respiratory, and laboratory variables
| All (n=1009) | No ACS-AMI (n=606) | ACS-AMI (n=403) | P | |
|---|---|---|---|---|
| ECG | ||||
| NIV starting | 14.3±2 | 14.1±2.2 | 14.5±1.5 | .001 |
| 1 h on NIV | 14.8±0.7 | 14.8±0.7 | 14.9±0.7 | .183 |
| HR,bpm | ||||
| NIV starting | 109±31 | 112±34 | 105±24 | .001 |
| 1 h on NIV | 98±31 | 98±20 | 98±19 | .815 |
| Systolic BP, mmHg | ||||
| NIV starting | 164±41 | 175±41 | 148±35 | <.001 |
| 1 hour on NIV | 145±21 | 151±22 | 137±16 | <.001 |
| Mean BP, mmHg | ||||
| NIV starting | 97±26 | 103±26 | 89±23 | <.001 |
| 1 h on NIV | 79±13 | 81±14 | 77±12 | <.001 |
| RR, breaths per min | ||||
| NIV starting | 37±5 | 37±5 | 37±4 | .353 |
| 1 h on NIV | 29±4 | 29±4 | 29±4 | .303 |
| Arterial pH | ||||
| NIV starting | 7.32±0.12 | 7.31±0.13 | 7.34±0.13 | <.001 |
| 1 h on NIV | 7.34±0.07 | 7.33±0.07 | 7.35±0.07 | .047 |
| PaCO2, mmHg | ||||
| NIV starting | 48±21 | 51±22 | 44±18 | <.001 |
| 1 h on NIV | 43±15 | 45±16 | 40±13 | <.001 |
| PaO2/FiO2, mmHg | ||||
| NIV starting | 129±33 | 130±34 | 127±32 | .143 |
| 1 h on NIV | 168±35 | 169±36 | 165±35 | .066 |
| HCO3–, mEq/L | ||||
| NIV starting | 24.5±4.4 | 24.7±4.5 | 24.1±4.2 | .026 |
| 1 h on NIV | 24.5±5.6 | 24.8±5.7 | 24.1±5.4 | .061 |
| Lactate, mmols/L | ||||
| NIV starting | 1.6±0.8 | 1.4±0.7 | 1.8±0.8 | <.001 |
| 1 h on NIV | 1.5±0.8 | 1.3±0.7 | 1.7±0.8 | <.001 |
| LVEF % | 37±12 | 39±12 | 33±10 | <.001 |
| CPK-Mb, ng/mL | 3 [2-21] | 2.1 [1.5-2.8] | 25 [17.4-42.5] | <.001 |
| Troponin I, ng/mL | 0.2 [0.1-10.3] | 0.08 [0.05-0.14] | 17 [5.6-47.8] | <.001 |
| NT-proBNP, pg/mL | 4357±2745 | 4103±2635 | 4732±2864 | .002 |
| C reactive protein, mg/dL | 30.4±17.1 | 25.9±11.9 | 37±20.9 | <.001 |
| Anemia | 246 (24.4) | 157 (25.9) | 89 (22.1) | .166 |
| Creatinine, mg/dL | ||||
| Initial | 1.3±0.7 | 1.3±0.8 | 1.3±0.7 | .322 |
| Maximum | 1.5±1.1 | 1.5±1.1 | 1.4±1.0 | .140 |
ACS-AMI, acute coronary syndrome-acute myocardial infarction; BP, blood pressure; CPK-Mb, creatine phosphokinase fraction Mb; ECG, electrocardiography; FiO2, inspiratory oxygen fraction; HCO3-, bicarbonate; HR, heart rate; LVEF, left ventricular ejection fraction; n, number; NIV, noninvasive ventilation; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PaCO2, carbon dioxide partial pressure; PaO2, oxygen partial pressure; RR, respiratory rate.
The data are expressed as mean±standard deviation or median [interquartile range].
Echocardiography was performed in 939 patients (ACS-AMI: 403 and non–ACS-AMI: 536), determination of CPK-Mb in 94 patients (ACS-AMI: 28 and non–ACS-AMI: 66); troponin I in 915 patients: (ACS-AMI: 375 and non–ACS-AMI: 540), C-reactive protein in 860 patients (ACS-AMI: 351 and non–ACS-AMI: 509) and NT-proBNP in 736 patients (ACS-AMI: 293 and non–ACS-AMI: 443).
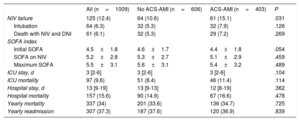
At the beginning of ventilatory therapy, a higher number of patients with ACS-AMI received CPAP (table 3). All other variables analyzed were similar between the 2 groups. Although NIV use was longer in patients without ACS-AMI, this difference was not statistically significant. Neither the number of patients with complications, nor most of them individually, showed differences between the 2 groups. Only 1 complication, pain of coronary etiology, was more frequent in patients with ACS-AMI during the period of NIV.
Ventilatory settings
| All (n=1009) | No ACS-AMI (n=606) | ACS-AMI (n = 403) | P | |
|---|---|---|---|---|
| Initial ventilatory mode | .031 | |||
| CPAP | 80 (7.9) | 39 (6.4) | 41 (10.2) | |
| Bilevel mode | 929 (92.1) | 567 (93.6) | 362 (89.8 | |
| Final ventilation mode | .187 | |||
| CPAP | 121 (12) | 66 (10.9) | 55 (13.6) | |
| Bilevel | 888 (86.4) | 540 (89.1) | 348 (86.4) | |
| Ventilator | .956 | |||
| BiPAP STD | 44 (4.4) | 26 (4.3) | 18 (4.5) | |
| BiPAP Vision | 922 (91.4) | 555 (91.6) | 367 (91.1) | |
| BiPAP V60 | 43 (4.3) | 25 (4.1) | 18 (4.5) | |
| IPAP, cmH2O | ||||
| Initial | 15.1±1.8 | 15.1±1.9 | 14.9±1.7 | .100 |
| Maximum | 16.2±2.4 | 16.2±2.6 | 16.2±2.3 | .941 |
| EPAP, cmH2O | ||||
| Initial | 7.1±0.8 | 7.1±0.9 | 7.1±0.8 | .198 |
| Maximum | 7.6±1.2 | 7.6±1.2 | 7.6±1.1 | .232 |
| FiO2.% | ||||
| Initial | 71±21 | 70±20 | 72±21 | .253 |
| Maximum | 74±21 | 73±21 | 75±21 | .320 |
| Leakage rate, L/min | ||||
| Mean | 32.7±7.4 | 32.8±7.2 | 32.6±7.7 | .580 |
| Minimum | 22.2±7.6 | 22.2±7.5 | 22.1±7.9 | .831 |
| High-flow oxygen | 33 (10.1) | 20 (10) | 13 (10.1) | .994 |
| NIV duration, h | 10 [6-20] | 12 [6-23] | 9 [6-20] | .182 |
| NIV complications | 158 (15.7) | 96 (15.8) | 62 (15.4) | .845 |
| Skin lesion | 123 (12.2) | 72 (11.9) | 51 (12.7) | .713 |
| Skin necrosis | 18 (1.8) | 8 (1.3) | 10 (2.5) | .172 |
| Eye irritation | 47 (4.7) | 27 (4.5) | 20 (5) | .708 |
| Claustrophobia | 45 (4.5) | 28 (4.6) | 17 (4.2) | .762 |
| Total intolerance | 10 (1) | 7 (1.3) | 3 (0.7) | .748 |
| Gastric distension | 21 (2.1) | 10 (1.7) | 11 (2.7) | .239 |
| Vomiting | 7 (0.7) | 5 (0.8) | 2 (0.5) | .709 |
| Bronchoaspiration | 1 (0.1) | 0 | 1 (0.2) | .309 |
| Respiratory infection | 5 (0.5) | 4 (0.7) | 1 (0.2) | .654 |
| Pneumothorax | 4 (0.4) | 2 (0.3) | 2 (0.5) | .654 |
| ACS | 12 (1.2) | 1 (0.2) | 11 (2.7) | <.001 |
| Mucous plugging | 2 (0.1) | - | 2 (0.1) | .492 |
ACS, acute coronary syndrome; ACS-AMI, acute coronary syndrome-acute myocardial infarction; cmH2O, centimeters of water; CPAP, continuous positive airway pressure; EPAP, positive airway expiratory pressure; FiO2, inspiratory oxygen fraction; IPAP, inspiratory positive airway pressure; n, number; NIV, noninvasive ventilation.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Neither the initial SOFA index score, nor subsequent scores during NIV or ICU stay differed between the groups (table 4). Although NIV failure (need for intubation or death during NIV with do not intubate order), mortality in the ICU, hospital and at 1 year, were slightly higher in the group with ACS-AMI, these differences were not statistically significant. There were 101 deaths (80.8%) in patients with NIV failure, and 56 deaths (6.3%) in patients with successful NIV (P <.001). The relationship between NIV failure and hospital mortality was observed both in patients with ACS-AMI (OR, 73.501; 95%CI, 33.364-161.918) and in non–ACS-AMI (OR, 57.474; 95%CI, 28.284-116.786).
Patient outcomes
| All (n=1009) | No ACS-AMI (n=606) | ACS-AMI (n=403) | P | |
|---|---|---|---|---|
| NIV failure | 125 (12.4) | 64 (10.6) | 61 (15.1) | .031 |
| Intubation | 64 (6.3) | 32 (5.3) | 32 (7.9) | .126 |
| Death with NIV and DNI | 61 (6.1) | 32 (5.3) | 29 (7.2) | .269 |
| SOFA index | ||||
| Initial SOFA | 4.5±1.8 | 4.6±1.7 | 4.4±1.8 | .054 |
| SOFA on NIV | 5.2±2.8 | 5.3±2.7 | 5.1±2.9 | .459 |
| Maximum SOFA | 5.5±3.1 | 5.6±3.1 | 5.4±3.2 | .489 |
| ICU stay, d | 3 [2-6] | 3 [2-6] | 3 [2-6] | .104 |
| ICU mortality | 97 (9.6) | 51 (8.4) | 46 (11.4) | .114 |
| Hospital stay, d | 13 [9-19] | 13 [9-13] | 12 [8-19] | .362 |
| Hospital mortality | 157 (15.6) | 90 (14.9) | 67 (16.6) | .478 |
| Yearly mortality | 337 (34) | 201 (33.6) | 136 (34.7) | .725 |
| Yearly readmission | 307 (37.3) | 187 (37.6) | 120 (36.9) | .839 |
ACS-AMI, acute coronary syndrome-acute myocardial infarction; DNI, do not intubate order; ICU, intensive care unit; NIV, noninvasive ventilation; SOFA, Sequential Organ Failure Assessment.
The data are expressed as No. (%), means±standard deviation, or median [interquartile range].
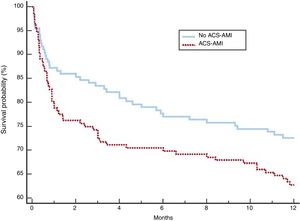
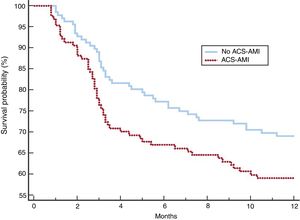
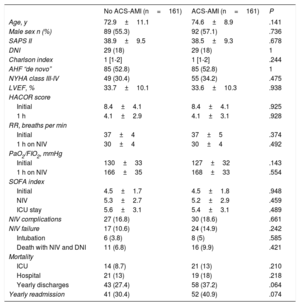
After adjustment, the 2 groups of patients showed a more balanced distribution of the variables (table 5). Although complications, NIV failure, ICU and hospital mortality were more frequent in the group with ACS-AMI, these differences were not significant. The increased risk of NIV failure in the ACS-AMI group was 4.3% (95%CI,−2.3 to 2.4) and that of hospital death was 5% (95%CI, −2.3 to 2.5). The 1-year mortality (figure 2) showed an adjusted hazard ratio of 1.456 (95%CI, 0.986-2.152) for the ACS-AMI group (P=.059). Hospital readmission per year is shown in figure 3. The hazard ratio adjusted for readmission in the ACS-AMI group was 1.467 (95%CI, 0.977-2.201) (P=.062).
Comparison of demographic, clinical, and outcome characteristics in the 2 groups of patients in the groups matched by propensity analysis
| No ACS-AMI (n=161) | ACS-AMI (n=161) | P | |
|---|---|---|---|
| Age, y | 72.9±11.1 | 74.6±8.9 | .141 |
| Male sex n (%) | 89 (55.3) | 92 (57.1) | .736 |
| SAPS II | 38.9±9.5 | 38.5±9.3 | .678 |
| DNI | 29 (18) | 29 (18) | 1 |
| Charlson index | 1 [1-2] | 1 [1-2] | .244 |
| AHF “de novo” | 85 (52.8) | 85 (52.8) | 1 |
| NYHA class III-IV | 49 (30.4) | 55 (34.2) | .475 |
| LVEF, % | 33.7±10.1 | 33.6±10.3 | .938 |
| HACOR score | |||
| Initial | 8.4±4.1 | 8.4±4.1 | .925 |
| 1 h | 4.1±2.9 | 4.1±3.1 | .928 |
| RR, breaths per min | |||
| Initial | 37±4 | 37±5 | .374 |
| 1 h on NIV | 30±4 | 30±4 | .492 |
| PaO2/FIO2, mmHg | |||
| Initial | 130±33 | 127±32 | .143 |
| 1 h on NIV | 166±35 | 168±33 | .554 |
| SOFA index | |||
| Initial | 4.5±1.7 | 4.5±1.8 | .948 |
| NIV | 5.3±2.7 | 5.2±2.9 | .459 |
| ICU stay | 5.6±3.1 | 5.4±3.1 | .489 |
| NIV complications | 27 (16.8) | 30 (18.6) | .661 |
| NIV failure | 17 (10.6) | 24 (14.9) | .242 |
| Intubation | 6 (3.8) | 8 (5) | .585 |
| Death with NIV and DNI | 11 (6.8) | 16 (9.9) | .421 |
| Mortality | |||
| ICU | 14 (8.7) | 21 (13) | .210 |
| Hospital | 21 (13) | 19 (18) | .218 |
| Yearly discharges | 43 (27.4) | 58 (37.2) | .064 |
| Yearly readmission | 41 (30.4) | 52 (40.9) | .074 |
ACS-AMI, acute coronary syndrome-acute myocardial infarction; AHF, acute heart failure; DNI, do not intubate order; FiO2, inspiratory oxygen fraction; HACOR, NIV failure prediction scale [H, heart rate. A, acidosis. C, consciousness. O, oxygenation. R, respiratory rate]; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NIV, noninvasive ventilation; NYHA, New York Heart Association; PaO2, oxygen partial pressure; RR, respiratory rate; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
In this large series of patients with AHF treated with NIV, the presence of AMI did not show a worse prognosis than AHF due to other causes.
The importance of AHF in hospitalized patients after an AMI is determined by 2 factors: on the one hand its high incidence and, on the other hand, by having a worse prognosis.24,25 The presence of ACS-AMI as the trigger for an AHF episode is highly variable. In our work, 5.4% of patients admitted with ACS-AMI had AHF that required NIV, considerably above the 0.4% of patients with STEMI treated with NIV in the American National Inpatient Sample database.26 In 40% of our patients with AHF, it was related to the presence of AMI, a value similar to the 32% reported by the FINN-AKVA study.11 In both observational and randomized controlled studies on patients with AHF treated with NIV, which included patients with AMI, the prevalence of ACS-AMI as the cause of the episode was highly variable, ranging from 17.2%27 to 72.9%17 in observational studies and between 5.5%28 to 70.4%29 in randomized studies. A worse outcome in patients with AMI who have AHF has been clearly established.6,26 More controversial is whether ACS-AMI as the trigger for the episode of AHF is associated with worse prognosis than other triggers. Tarvasmäki et al.11 reported higher hospital mortality in the Finn-AKVA Finnish study in patients with AHF due to AMI, but 5-year mortality did not differ between the 2 groups. In a study conducted over 11 years, Figueras et al.12 analyzed 806 patients with acute cardiogenic lung edema: hospital mortality did not differ between patients with and without coronary heart disease, although at 4 years, mortality was higher in the first group. Among patients with AHF treated with NIV, the results remain contradictory. In a series of 29 patients with pulmonary edema treated with NIV, ventilatory therapy was successful in all 5 cases with AMI.27 However, Rustherholz et al. showed that the presence of AMI was related to the failure of NIV.13 In our series, the group with ACS-AMI had a significant increase in NIV failures, and higher mortality in the ICU, hospital and at 1 year, but without reaching statistical significance, similar to the results of Lazzeri et al.16 In that study, mortality in the ICU was particularly high, 38.5% in patients with AMI and 30.8% in the group without infarction, well above the mortality in our study (11.4% and 8.4%, respectively), probably because of the inclusion of patients with cardiogenic shock. This lack of association between ACS and higher mortality has been reported by other studies. In a series of 118 patients with AHF treated with NIV, including patients in cardiogenic shock, the presence of AMI as the cause of heart failure was not related to a worse prognosis.17 Yamamoto et al.15 carried out a retrospective study on 206 patients with acute pulmonary edema treated with NIV, 53 of them with AMI. Although the rate of endotracheal intubation and mortality in the ICU and in-hospital was higher in the group with AMI, the differences did not reach statistical significance.15 To analyze more precisely the relationship between the presence of ACS-AMI and worse outcome, matching was carried out using propensity score analysis. This resulted in 2 groups of patients with more balanced prognostic variables. Although the rate of NIV failure, in-hospital and 1-year mortality, and hospital readmissions were more frequent in the group with ACS-AMI, the differences between the 2 groups did not reach statistical significance. The term NIV failure used in this study was a combined endpoint of the need for intubation and death in the ICU or on the ward in patients without resolution of the AHF episode. With this definition, we attempted to prevent the bias caused by patients with a no-intubation order in whom intubation is ruled out.30 The absence of resolution of the AHF episode is a poor prognostic factor both in patients with and without limitation of therapeutic effort.14,17,29
On analysis of the characteristics of patients with and without ACS-AMI, multiple variables showed differences between the 2 groups. Our series showed a higher percentage of men in the group with ACS-AMI, a finding that was also observed in other case series, but without reaching statistical significance.11,15,16 Among the clinical antecedents, a history of acute or chronic heart failure was more frequent in patients without ACS-AMI, which conferred a worse New York Heart Association functional class. This finding, as well as a higher percentage of patients with a history of chronic atrial fibrillation, has been reported by other authors.11 In relation to respiratory physiological variables, patients without ACS-AMI had higher PaCO2 levels and lower pH, with a similar respiratory rate and oxygenation rate. This could be related to a higher percentage of patients with acute on chronic heart failure in this group of patients. Patients with ACS showed a significantly lower LVEF value. The mean LVEF of the patients analyzed was 37%, similar to that shown in the series by Yamamoto et al.,15 and clearly lower than that of the patients in the FINN-AKVA registry.11 These differences can be explained by the lower severity of the patients analyzed. In the FINN-AKVA study, only 24% of patients required noninvasive respiratory support.11
The ideal ventilatory mode in patients with AHF is not clear. Both the application of CPAP and support pressure or bilevel mode have been widely used. Randomized controlled studies comparing the 2 modalities showed no differences in the rate of endotracheal intubation or mortality.9 In this study, the mode initially used was bilevel, which was switched to CPAP mode in the presence of discomfort or intolerance; in studies by other authors,15 the initial mode was CPAP, which was switched to NIV in the absence of clinical improvement. The faster response to NIV therapy compared with CPAP with an earlier improvement in clinical symptoms can be considered as an important factor when using one or other noninvasive support mode, as well as patient admission to the ICU.31 Complications related to NIV were similar in the 2 groups. The complications related to NIV are frequent and are related to its duration.14 Neither the duration nor the values of the settings, nor the levels of leakage through the mask differed between the 2 groups. Only new ischemic pain was more frequent in the group with ACS-AMI.
Strengths and weaknessesThis study has some strengths and weaknesses. This is a large prospective cohort study of patients with AHF followed up for 1 year that analyzed multiple clinical, physiological variables. It was performed in the “real world” and included all patients consecutively treated with NIV. In addition, the study included a propensity analysis to further specify the relationship between the presence of ACS-AMI and patient outcomes.
A weakness of this study is that this is an observational study and therefore subject to multiple biases when evaluating the results of the efficacy of the ventilatory technique. It was also carried out over a long period with the consequent changes in some of the definitions on the diseases analyzed; however, we attempted to overcome this weakness by homogenizing patients and analyzing the recorded data. In addition, the long study period led to variation in the sociodemographic, clinical and outcome characteristics of the patients, which may be analyzed in future works. This study was carried out in an ICU with extensive experience in NIV, both in cardiac and pulmonary diseases, with perfectly trained medical and nursing staff, in addition to having a wide range of devices, ventilators and interfaces, which may affect extrapolation of the results to other areas with less experience or resources. Finally, despite the huge number of variables collected, we may have omitted some important variables. Despite the limitations mentioned, we believe that the results are perfectly valid.
CONCLUSIONsThe presence of ACS-AMI as a cause of AHF did not influence the outcome of patients with acute respiratory failure requiring noninvasive respiratory support. Complications derived from NIV were not more frequent in patients with ACS-AMI.
- -
NIV is an important strategy in the management of acute heart failure by reducing the need for endotracheal intubation and mortality. ACS-AMI is a frequent trigger for an AHF episode. Most clinical trials evaluating the role of NIV excluded patients with AHF triggered by ACS-AMI. The role of NIV in the treatment of AHF in patients with ACS-AMI is not well studied.
- -
The presence of ACS-AMI as the cause of AHF did not confer a worse prognosis in patients with AHF requiring respiratory support. Mortality or hospital readmission at 1 year was related to the presence of ACS-AMI. Complications derived from NIV were not more frequent in patients with ACS-AMI.
All authors have contributed to the study conceptualization, data curation, formal analysis, investigation, methodology, validation, writing and reviewing. A. Carrillo-Alcaraz also contributed to project administration, software, and supervision.
CONFLICTS OF INTERESTNone.