Percutaneous paravalvular leak closure is a complex procedure with varying success rates; the lack of closure devices specifically designed for this purpose has hampered this technique. The characteristics of the Amplatzer Vascular Plug III appear to be well suited for paravalvular leak closures; however, the available data are limited to case reports or small series of patients. The aim of this study was to analyze the feasibility and efficacy of paravalvular leak with this device.
MethodsThe immediate and 90-day safety and efficacy of mitral and aortic paravalvular leak closures performed with this device at our hospital were analyzed.
ResultsPercutaneous repair of 34 paravalvular leaks (27 mitral, 7 aortic) was attempted in 33 patients. The device was successfully implanted in 93.9% (in 2 patients, a second planned procedure was needed), and successful closure (defined as regurgitation reduction ≥ 1 grade) was achieved in 90.9% of patients. Complications included emergency surgery due to disc interference (n=1) and blood transfusion (n=3). There were no reports of procedure-related death, myocardial infarction, or stroke. At 90 days, survival was 100%, and 90.3% of patients showed significant clinical improvement; 4 patients developed vascular complications (pseudoaneurysm).
ConclusionsMitral and aortic paravalvular leak closure with the Amplatzer Vascular Plug III is feasible and safe, with high clinical and echocardiographic success rates.
Keywords
Paravalvular leak (PVL) is a relatively common complication of valve replacement surgery.1 Although most PVLs are small and asymptomatic, 2% to 5%2–4 are clinically relevant and associated with major complications, such as heart failure (HF), hemolytic anemia, arrhythmias, and infective endocarditis.5,6
Classically, the treatment of choice for patients with symptomatic PVLs was surgical reoperation, either repairing the valvular dehiscence or replacing the prosthesis.1 Recently, percutaneous treatment of PVLs has emerged as a therapeutic alternative for high–surgical-risk patients.7 In general, this procedure is associated with satisfactory short- and long-term results.2,8 However, the success rate for the technique varies considerably.2,9 A number of devices not specifically designed for this task have been used to treat PVLs. Due to its characteristics and design, the Amplatzer Vascular Plug III (AVP III) (St. Jude Medical) is an ideal device for this procedure and has recently been used off-label for PVL closure. To date, the results available with this device have been limited to small series or isolated cases.10–13
The aim of this study was to evaluate the outcomes of PVL closure with the AVP III device in one of the largest PVL closure series published to date.
METHODSPopulationPercutaneous closure was performed in 33 patients on 34 consecutive PVLs between July 2009 (first case) and March 2013. All had PVLs that produced serious symptomatic regurgitation (HF and/or hemolytic anemia requiring periodic red blood cell transfusion). Each patient's case was discussed in the medical-surgical session and was considered eligible for percutaneous PVL closure. Patients with single or multiple PVLs that, as a whole, affected more than a third of the prosthetic ring circumference were considered ineligible for percutaneous closure. All patients gave written informed consent for the procedure.
Definition of the Variables AnalyzedPVL was defined as the presence of a regurgitation jet on Doppler echocardiography that originated between the border of the prosthetic ring and the surrounding native tissue. The American Society of Echocardiography14 recommendations were used to define regurgitation severity. The site of the mitral PVLs was defined according to the classification previously proposed by Cortes et al,15 and the site of aortic PVLs was defined using the classification adopted by Ruiz et al.2
HF was diagnosed according to the classical Framingham criteria, and functional status was assessed at baseline and during follow-up according to the New York Heart Association classification. Hemolytic anemia was defined as plasma hemoglobin ≤ 14g/dL in men or ≤ 12g/dL in women, hemolytic profile (lactate dehydrogenase ≥ 600 U/L, haptoglobin ≤ 10mg/dL), and red blood cell transfusion within the past 6 months.
Technical success was defined as proper device implantation in the PVLs without interference with the prosthetic discs or a need for emergency conversion to conventional surgery. The procedure was considered to be successful if, in addition to the above criteria, the echocardiogram showed a decrease ≥ 1 degree in valvular regurgitation.
Procedure-related events were considered to be complications that occurred during the procedure or within the following 24 h (procedure-related death, cardiovascular death, acute cerebrovascular event, myocardial infarction, cardiac tamponade, vascular complications at the vascular access that required surgery, or red blood cell transfusion and emergency conversion to traditional surgery).
Procedure Technique and CharacteristicsIn all patients, the procedure was performed under general anesthesia and guided with transesophageal echocardiography (TEE), 2-dimensional in the first 6 patients and real-time 3-dimensional in all others (81.8%).
In all patients, the percutaneous closure of aortic PVLs was done by a retrograde approach, using previously described techniques.2,9,16,17 Briefly, a catheter with a hydrophilic guidewire is advanced to the aorta and used to cross the PVL; once the leak is crossed with the hydrophilic guidewire, the catheter is advanced and, once in the ventricle, switched to a guidewire with a larger support to allow the device delivery sheath to be advanced through the leak. The device is introduced over this sheath and implanted by sequentially delivering the distal disc in the ventricular aspect and the proximal disc in the aortic aspect of the leak.
Mitral PVL closure was performed using a retrograde or anterograde route, depending on patient anatomy and PVL site, using previously described techniques.2,9,16 Briefly, in the anterograde approach, transseptal puncture is performed and the PVL is crossed with a guidewire from the left atrium to the left ventricle, whereas in the retrograde approach, the guidewire is passed from the left ventricle through the leak to the left atrium. An arteriovenous loop was established in all patients (using a loop to snare the guidewire in the left atrium in the retrograde approach and in the aorta in the anterograde approach) and the delivery sheath was advanced from atrium to ventricle.
In patients with mitral PVLs and a 2-disc aortic prosthesis, once an anterograde access was attempted without success, the retrograde approach was used, crossing the central opening of the aortic prosthesis with a hydrophilic catheter and a straight guidewire, and the same steps were followed as in other retrograde approaches.
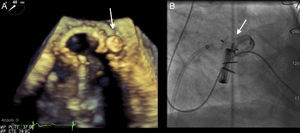
The AVP III device was used in all patients. This is an oval nitinol device with a height of 6.5 mm, and sizes that vary from 4 mm to 14 mm along the long axis and 2 mm to 5 mm along the short axis. The delivery sheaths vary from 4 Fr to 7 Fr, depending on the device size (Figure 1).
The size of the PVL and, therefore, the size of the device to be implanted, was assessed by TEE. In patients who underwent 3-dimensional TEE, the color images acquired in 3-dimensions were used. The anatomic characteristics (area, width, and length) of each PVL were measured from the image of the complete volume using the multiplanar reconstruction tool, such that the longitudinal and coronal planes crossed in the area of the dehiscence visualizing regurgitant flow, whereas the transverse plane or short axis was used to measure the regurgitant orifice at the point of origin.
After transseptal puncture, all patients were given intravenous heparin to achieve an activated clotting time of 250s and were started oral anticoagulation after the procedure; heparin therapy was maintained until adequate levels were obtained with oral anticoagulants.
Clinical and Echocardiographic Follow-upPatients were checked by the cardiology outpatient clinic and TEE was performed 90 days after the procedure. The following variables were collected: New York Heart Association functional class, need for red blood cell transfusion, all-cause death, cardiovascular death, acute cerebrovascular events, myocardial infarction, need for cardiac surgery, vascular complications requiring red blood cell transfusion or surgery and degree of valvular regurgitation.18
Clinical success in follow-up was considered to be proven clinical improvement ≥ 1 degree in New York Heart Association functional class in the 90 days after the closure procedure.
Statistical AnalysisA retrospective descriptive study was performed. Continuous variables are presented as mean (standard deviation) and qualitative variables, as absolute number and percentage. The statistical analysis was performed using SPSS 16.0 (IBM SPSS Statistics).
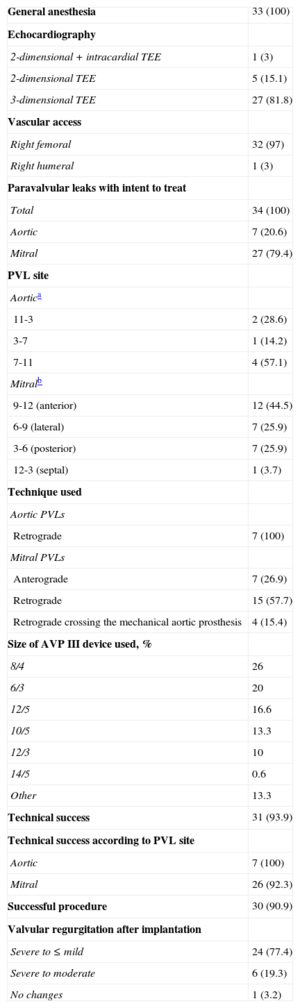
RESULTSDuring the period analyzed, percutaneous closure was performed in 34 PVLs: 7 in the aortic position and 27 at the mitral level. In 1 patient, 2 PVLs were closed at different sites during the same procedure. The baseline demographic and clinical characteristics are summarized in Table 1.
Demographic and Clinical Characteristics of the Patients
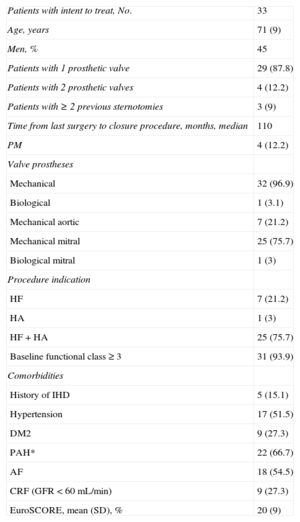
| Patients with intent to treat, No. | 33 |
| Age, years | 71 (9) |
| Men, % | 45 |
| Patients with 1 prosthetic valve | 29 (87.8) |
| Patients with 2 prosthetic valves | 4 (12.2) |
| Patients with ≥ 2 previous sternotomies | 3 (9) |
| Time from last surgery to closure procedure, months, median | 110 |
| PM | 4 (12.2) |
| Valve prostheses | |
| Mechanical | 32 (96.9) |
| Biological | 1 (3.1) |
| Mechanical aortic | 7 (21.2) |
| Mechanical mitral | 25 (75.7) |
| Biological mitral | 1 (3) |
| Procedure indication | |
| HF | 7 (21.2) |
| HA | 1 (3) |
| HF+HA | 25 (75.7) |
| Baseline functional class ≥ 3 | 31 (93.9) |
| Comorbidities | |
| History of IHD | 5 (15.1) |
| Hypertension | 17 (51.5) |
| DM2 | 9 (27.3) |
| PAH* | 22 (66.7) |
| AF | 18 (54.5) |
| CRF (GFR<60 mL/min) | 9 (27.3) |
| EuroSCORE, mean (SD), % | 20 (9) |
AF, atrial fibrillation; CRF, chronic renal failure; DM2, type 2 diabetes mellitus; GFR, glomerular filtration rate; HA, hemolytic anemia; HF, heart failure; IHD, ischemic heart disease; PAH, pulmonary arterial hypertension; PM, permanent pacemaker; SD, standard deviation.
Unless otherwise indicated, data are expressed as No. (%) or mean (standard deviation).
*Pulmonary artery systolic pressure estimated by echocardiography ≥ 40 mmHg.
Men accounted for 45% of patients, and the mean age was 71 (9) years. The most common indication of the procedure was the concomitant presence of HF and hemolytic anemia (75.7%). In 21.2% ofpatients, the closure indication was exclusively HF, and only 1 patient was referred for closure due to symptomatic hemolytic anemia and a need for periodic transfusions. The treated population had high comorbidity and high surgical risk. The estimated mean logistic EuroSCORE before the procedure was 20% (9%).
Implants, Complications, and Immediate OutcomesIn aortic PVLs, the retrograde approach was used in all patients. Among mitral PVLs, the retrograde approach was used in 57.7% of patients, the anterograde approach in 26.9%, and the retrograde approach crossing the mechanical aortic prosthesis to close the leak in 15.4% (Figure 2). In 1 patient, 2 contiguous devices were implanted using the anterograde approach to close 1 PVL. The procedure characteristics are summarized in Table 2.
Procedure Characteristics
| General anesthesia | 33 (100) |
| Echocardiography | |
| 2-dimensional+intracardial TEE | 1 (3) |
| 2-dimensional TEE | 5 (15.1) |
| 3-dimensional TEE | 27 (81.8) |
| Vascular access | |
| Right femoral | 32 (97) |
| Right humeral | 1 (3) |
| Paravalvular leaks with intent to treat | |
| Total | 34 (100) |
| Aortic | 7 (20.6) |
| Mitral | 27 (79.4) |
| PVL site | |
| Aortica | |
| 11-3 | 2 (28.6) |
| 3-7 | 1 (14.2) |
| 7-11 | 4 (57.1) |
| Mitralb | |
| 9-12 (anterior) | 12 (44.5) |
| 6-9 (lateral) | 7 (25.9) |
| 3-6 (posterior) | 7 (25.9) |
| 12-3 (septal) | 1 (3.7) |
| Technique used | |
| Aortic PVLs | |
| Retrograde | 7 (100) |
| Mitral PVLs | |
| Anterograde | 7 (26.9) |
| Retrograde | 15 (57.7) |
| Retrograde crossing the mechanical aortic prosthesis | 4 (15.4) |
| Size of AVP III device used, % | |
| 8/4 | 26 |
| 6/3 | 20 |
| 12/5 | 16.6 |
| 10/5 | 13.3 |
| 12/3 | 10 |
| 14/5 | 0.6 |
| Other | 13.3 |
| Technical success | 31 (93.9) |
| Technical success according to PVL site | |
| Aortic | 7 (100) |
| Mitral | 26 (92.3) |
| Successful procedure | 30 (90.9) |
| Valvular regurgitation after implantation | |
| Severe to ≤ mild | 24 (77.4) |
| Severe to moderate | 6 (19.3) |
| No changes | 1 (3.2) |
AVP III, Amplatzer Vascular Plug III; PVL: paravalvular leak; TEE, transesophageal echocardiography.
Unless otherwise indicated, data are expressed as No. (%).
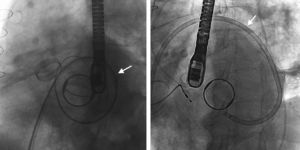
In 4 of 33 patients, the implant was initially unsuccessful. In 1 patient, the device moved after delivery and interfered with the mitral prosthetic discs, it could not be snared again and the patient underwent emergency surgery. In 1 patient, it was impossible to cross the PVLs (anterior PVL in biological prosthesis with no radiopaque structure) and the patient was scheduled for surgery. In 1 patient, transseptal puncture (atrial septal aneurysmal) was initially impossible and, therefore, the procedure was repeated using a steerable catheter (Agilis, St. Jude Medical) without complications (Figure 3). In 1 patient, the device interfered with the mitral discs by the angle of access to the PVLs and was not delivered; the procedure was rescheduled and repeated using a higher transseptal puncture with the aid of a steerable catheter, which allowed the device to be delivered without complications. In summary, technical success was achieved in 93.5% of patients, although 2 of them required a second planned procedure. In all patients except 1, implant success was accompanied by a decrease ≥ 1 1 regurgitation grade (procedure success, 90.9%).
Procedure-related complications occurred in 4 patients. As mentioned, 1 patient required surgery and 3 required red blood cell transfusion in the first 24 h after the procedure due to hematoma in the puncture region; all had hemolytic anemia and low hemoglobin levels before the procedure, and transfusions were given to aid recovery. There were no reports of death, acute myocardial infarction, stroke, or need for vascular surgery.
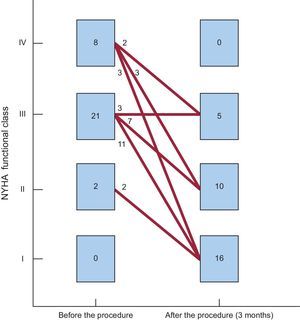
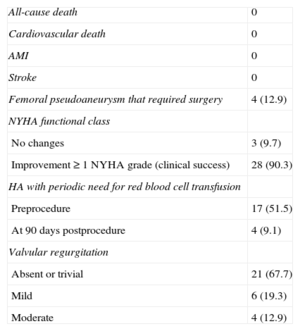
Clinical and Echocardiographic Follow-up at 90 daysIn 28 of 31 (90.3%) patients who received the device, an improvement ≥ 1 degree in functional class (clinical success at 90 days) was achieved. Only 3 patients experienced no improvement in functional class after the procedure (Figure 4). In addition, the need for periodic transfusions was significantly lowered with the closure procedure, from 51.5% to 9.1% (Table 3).
Clinical and Echocardiographic Follow-up at 90 Days
| All-cause death | 0 |
| Cardiovascular death | 0 |
| AMI | 0 |
| Stroke | 0 |
| Femoral pseudoaneurysm that required surgery | 4 (12.9) |
| NYHA functional class | |
| No changes | 3 (9.7) |
| Improvement ≥ 1 NYHA grade (clinical success) | 28 (90.3) |
| HA with periodic need for red blood cell transfusion | |
| Preprocedure | 17 (51.5) |
| At 90 days postprocedure | 4 (9.1) |
| Valvular regurgitation | |
| Absent or trivial | 21 (67.7) |
| Mild | 6 (19.3) |
| Moderate | 4 (12.9) |
AMI, acute myocardial infarction; HA, hemolytic anemia; NYHA, New York Heart Association.
The data are presented as No. (%).
Complications occurred during follow-up in 4 (12.9%) patients, all of vascular etiology (femoral pseudoaneurysm). Two of them were patients who underwent closure for aortic PVL with an 8-Fr delivery sheath, which could have favored the appearance of pseudoaneurysm. In the other 2 patients (mitral PVL closures), the left femoral access was used to perform the arteriovenous loop with 6-Fr introducers; in 1 patient, closure was performed with a device and in the other, manual compression. Both patients presented obesity as a risk factor for vascular complications. Traditional surgery was performed in 3 patients, and percutaneous treatment with thrombin was chosen in the other, all with good outcome. No deaths, myocardial infarctions, strokes, or need for cardiac surgery were recorded during follow-up (Table 3).
In the follow-up TEE, valvular regurgitation was undetectable or trivial in 67.7% of patients and mild in 19.3%. Only 4 (12.9%) patients showed moderate valvular regurgitation (Table 3).
DISCUSSIONThe main conclusions of our experience in PVL closure with the AVP III device are that: a) percutaneous closure of PVL is a safe procedure with a low rate of serious complications; b) the procedure has a high technical success rate, and c) the short-term results are favorable in mitral and aortic PVL closure, with significant improvement in the degree of regurgitation, New York Heart Association functional class, and need for transfusion.
Although the exact percentage of PVLs after valvular surgery is unknown because it varies considerably among series,19,20 some studies have stated that the presence of serious symptomatic PVLs is associated with lower morbidity and mortality if treated invasively compared with conservative management.21
Traditionally, PVL has been treated by surgery; however, this type of surgery is associated with a considerable risk of serious complications and need for reoperation. In addition, the published results vary.3,22–24 In clinical practice, many patients with symptomatic PVLs do not undergo a procedure due to their multiple comorbidities and the high estimated surgical risk, with the poor prognosis that this confers.21 At this stage, the use of a less invasive technique, such as percutaneous closure, is presented as a very attractive therapeutic alternative for the treatment of these patients.
Since the first procedure used for percutaneous closure of PVL was described,25 there has been a sharp rise in interest in the technique and the number of patients treated. The success and complication rates differ substantially, according to the series. This variation could be explained by differences in PVL morphology and size, and accesses and techniques used for treatment. In addition, the experiences published to date include only a few patients and usually reflect the data from a single site, making it difficult to extrapolate the outcomes.
The oval or half-moon morphology of most PVLs makes it difficult to find a specific device that adapts to these defects. For this reason, a number of devices not specifically designed for this task have been used to treat PVLs.15,25–29 Because of its characteristics and design, the AVP III device is ideal for PVL closure. However, the experience published with this device mainly consists of a few isolated cases and small series.10,12,30–32
As in previously published PVL percutaneous closure series,2,16,33 our study found that the most common indication for the procedure was concomitant HF and hemolytic anemia, and the treatment population had elevated comorbidity and high surgical risk.
In our series, the technical success was 93.9%, slightly higher than that of the largest series, recently published by Ruiz et al2 (86%) and Sorajja et al16 (89%). However, these series did not use the AVP III device and the access was transapical in many of the patients, which limits the comparison.
The high percentage of success has several explanations. In our series, all procedures were guided with TEE (real-time 3-dimensional TEE in most). In this regard, the participation of echocardiography specialists, who are familiar with the visualization of these defects and closure devices, is probably one of the key aspects to achieving a high success rate. Echocardiographic diagnosis of PVL is often complex.34,35 The incorporation of 3-dimensional TEE allowed the entire prosthetic valve to be visualized and enhanced PVL definition and characterization.36 Echocardiography has also acquired a major role in the closure procedure, as it is used to guide the operator during the various phases of the intervention, including the choice of atrial transseptal puncture site, the guidewire for the catheter and the device toward PVLs, the choice of the closure device, and the immediate assessment of the closure outcome.37,38 The series we present is relatively recent (2009-2013), which has allowed patients to benefit from technical advances made in the last few years and from the experience of other authors who have described the potential complications of the technique and their solutions; in addition, the main operator had previously performed or cooperated in this type of surgery at other sites, which may have favorably influenced the outcomes presented because the series did not cover the entire learning curve. Lastly, the use of the AVP III device in all cases may have influenced the outcomes. The features of the device make it more readily suitable for PVLs than other, previously used devices. The Amplatzer ASD Occluder device (St. Jude Medical) has a large distance between the waist and the discs (12-14 mm in most devices), which may increase interference with the prosthetic discs; the first-generation Amplatzer PDA Occluder (St. Jude Medical) had only 1 retention skirt, which could increase the risk of embolization; the Vascular Plug II and the VSD Occluder (St. Jude Medical) are round, and thus might be suitable for the few cases in which the PVLs are round. Although there are few data on the AVP III, the results are promising; Nietlispach et al,12 who described the initial experience with this device, obtained technical success in 100% of the 5 patients in whom it was implanted, Smolka et al11 reported 90% success in 11 patients, and Ozkan et al10 in 100% of 3 patients.
As in other series, most of the complications associated with the procedure were vascular. All patients were receiving acenocoumarol therapy, which could have influenced the course of these complications. There was no procedure-related death, myocardial infarction, or stroke. In the 90-day follow-up, survival was 100%, and 90.3% of patients had ≥ 1 degree of improvement in functional class.
LimitationsThis is a single-center descriptive study and, therefore, the conclusions may not be applicable to other settings. The study was not randomized and, therefore, the efficacy of the AVP III was not compared with that of other devices. The small number of patients made it difficult to draw conclusions on the factors that influenced the appearance of vascular complications. The follow-up presented is short term; hence, the medium- and long-term clinical and echocardiographic repercussions of this procedure with the AVP III device are unknown.
CONCLUSIONSPercutaneous PVL closure with the AVP III device in high-surgical-risk patients is a safe and effective technique that is clinically and echocardiographically successful in the short term.
CONFLICTS OF INTERESTSDr. Cruz Gonzalez has a consulting agreement with St. Jude Medical. All other authors declare no conflict of interests.