To assess the clinical characteristics of patients with atrial fibrillation in the primary care setting.
MethodsThis was a 2-phase, cross-sectional, multicenter study: phase A assessed the proportion of atrial fibrillation patients assisted in primary care over 5 days; phase B analyzed atrial fibrillation patients’ clinical characteristics and management.
ResultsIn phase A, 119 526 subjects (age 52.9 [15.2] years; 40.9% male) received primary care in participating centers; 6.1% had atrial fibrillation. This proportion increased with age, hypertension, and male sex. In phase B, we analyzed 3287 atrial fibrillation patients (age 71.9 [10.1] years; 52.3% male). Risk factors were hypertension (92.6%), hypercholesterolemia (70.6%), related cardiovascular disease, heart failure (21.3%), and ischemic heart disease (20.9%). Permanent atrial fibrillation was the most frequent type of atrial fibrillation (45.3%). Age and cardiac and renal diseases were related to permanent atrial fibrillation development. Although more than two-thirds of patients had a CHADS2 score ≥2, about one-third of them were not taking anticoagulants; by contrast, 46.8% of patients with CHADS2=0 were taking oral anticoagulants.
ConclusionsIn primary care, 6.1% of patients had atrial fibrillation. Patients with atrial fibrillation had high comorbidity. Anticoagulant treatment is far from optimal for atrial fibrillation patients in primary care.
Keywords
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice and has significant clinical and prognostic implications.1 Given the close relationship between AF and age, an increase in the prevalence of AF is expected in the coming years. For example, the ATRIA (AnTicoagulation and Risk Factors in Atrial Fibrillation) study noted that while there are currently 2.2 million people in the United States with AF, this is expected to increase to 5.6 million by 2050.2
Moreover, AF is not static but can develop over time. It has been observed that more than three-quarters of those with paroxysmal AF develop permanent AF after 14 years of follow-up, and about 20% of patients with recurrent AF develop permanent AF after 4 years.3, 4 The AF leads to electrical, and later structural, atrial remodeling, which can result in permanent AF. Although a trigger factor can be identified initially, in later stages a substrate leads to AF being sustained over time.5 It is therefore important to identify those factors or situations that facilitate AF development and persistence. Proper treatment could potentially reduce the likelihood of further recurrences or prevent AF from becoming permanent.
Different studies have identified several independent risk factors for developing recurrent or permanent AF. These include ischemic heart disease, heart failure, valvular heart disease, and high blood pressure.6 From a population point of view, hypertension is the most important risk factor for developing AF. For example, the CARDIOTENS study showed that in Spain 66% of AF patients had a history of hypertension.7, 8
Mortality in AF patients is about double that for those in sinus rhythm.1 The most serious complication of AF is the development of a stroke, both for short-term prognosis and for the disabling effects it produces. Since the thromboembolic risk is the same, regardless of the type of AF (paroxysmal, persistent, or permanent) and whether it is symptomatic or not, it is important to know whether physicians in clinical practice adequately assess the thromboembolic risk of AF patients and whether the antithrombotic therapy is appropriate.9
The primary care (PC) physician plays a key role in the management of AF patients. Although some studies have examined the prevalence of AF in different settings, including PC,7, 8, 10, 11 for Spain we have little detailed data analyzing the clinical characteristics of patients treated in PC for AF, its prevalence, its relationship with cardiovascular risk factors, the factors related to evolution towards permanent AF, or its clinical management in this setting. The Val-FAAP study (for the characterization and evaluation of AF patients treated in PC) was implemented to investigate the clinical characteristics of AF patients in PC. Secondary objectives were to estimate the prevalence of AF in PC consultations, describe the characteristics of patients with AF and hypertension, determine which variables were associated with progression to permanent AF, and assess the antithrombotic treatment used according to the CHADS2 score.
MethodsThis is a national, descriptive, epidemiological, cross-sectional study developed in the context of clinical practice. The study was designed in 2 simultaneous phases: phase A was to determine the proportion of subjects with AF in PC by counting all patients who were seen in PC over 5 days, and assessing the history of AF as documented by electrocardiogram results in medical records. Phase B described the characteristics of patients with a previous diagnosis of AF. In this phase, each researcher agreed to include a total of 4 patients who fulfilled the inclusion criteria: patients of both sexes, ≥18 years of age, previously diagnosed with AF by electrocardiography, and who had given informed consent in writing. The study was approved by the Fundació Jordi Gol i Gurina (IDIAP) Ethics Committee. It was conducted by 836 PC physicians throughout Spain. To ensure that the study was fair and representative of the whole country, the number of physicians chosen in each province was proportional to the number of inhabitants of the province; invited researchers were selected randomly. Physicians agreed to participate in the study after being informed in detail about the study objectives and methodology.
Patients were recruited between September 2009 and May 2010. A single visit was arranged to collect demographic/anthropometric and clinical data, as well as data from several additional tests (laboratory, electrocardiogram, and echocardiogram) performed 6 months prior to the visit. In addition, data were collected on the date of diagnosis as well as detailed patient characteristics from the visit immediately prior to the diagnosis of AF (prediagnosis information). The AF type was classified both at the time of diagnosis (whether patient were diagnosed at the visit [de novo] or referred with a previous diagnosis of paroxysmal, persistent, or permanent AF) and at the subsequent visit, according to the clinical guidelines in effect at the time of the study (2006).1 The AF was identified as new onset (first episode of symptomatic AF or first diagnosis of asymptomatic AF), paroxysmal (spontaneous recovery ≤7 days, and often <24h), persistent (lasting >7 days with no spontaneous recovery, reversed by pharmacological or electrical cardioversion), or permanent (underlying rhythm was AF and restoring sinus rhythm was either not possible or not indicated.)
Regarding definition of the different variables, overweight was defined as a body mass index between 25kg/m2 and 29.9kg/m2 and obesity ≥30kg/m2. A patient was considered to have dyslipidemia with one of the following indicators: total cholesterol >200mg/dL, low-density lipoprotein cholesterol >130mg/dL, high-density lipoprotein cholesterol <40mg/dL, triglycerides >150mg/dL, or receiving lipid-lowering therapy. For smoking, patients were classified as smokers, exsmokers <1 year, exsmokers ≥1 year, or nonsmokers. Physical activity was classified as sedentary (if the patient did no weekly exercise), moderate (some exercise 2 or 3 times a week) or active (playing sports or exercising more than 3 times a week). Renal failure was considered with an estimated glomerular filtration rate <60ml/min/1.73m2. Family history of premature cardiovascular disease was defined as the presence in first degree relatives of ischemic heart disease, cerebrovascular disease, or peripheral artery disease at <55 years for males and <65 years for women.
Left ventricular hypertrophy was defined according to the European societies of hypertension and cardiology guidelines 2007,12 electrocardiographic criteria (Sokolow SV1+RV5-6>38mm and/or Cornell product >2.440mm/ms), or echocardiography (left ventricular mass ≥125g/m2 in men or ≥110g/m2 in women).12
The risk of stroke was evaluated using the CHADS2 score. This scale assigns a value of 1 point for each of the following risk factors: congestive heart failure, hypertension, age >75 years, diabetes mellitus, and 2 points for having a previous stroke. According to the scale, a value of zero is considered low risk, 1 is moderate risk, and ≥2 is high risk.1
Calibrated mercury sphygmomanometers or semi-automatic apparatus, as available, were used for measuring blood pressure. The patient was given 5min of rest before 3 measurements were performed with the patient sitting; the average of the 3 determinations was taken as the result. Hypertensive patients were considered to have adequately controlled blood pressure if they met the European societies of hypertension and cardiology guidelines criteria (ESH-ESC) 200712: <140/90mmHg in the general hypertensive population or <130/80mmHg in diabetes or renal failure patients.
Statistical AnalysisBecause this was a descriptive study, sample size was calculated from a representative sample of the Spanish population seen in PC for AF. The CARDIOTENS7 study found a prevalence of 4.80% for AF in PC units. A sample of 3451 patients was calculated to yield results representative of AF patients in Spain with an error rate of 0.9%.
The continuous variables were defined as mean (standard deviation), and qualitative variables as absolute and relative frequency. Statistical tests were performed depending on the nature of the variables. The relationship between categorical variables was analyzed using the chi-square test; if more than 20% of the cells had an expected frequency lower than 5, the Fisher's exact test was used. The McNemar test was used for comparison of paired samples; preexisting cardiovascular risk factors at diagnosis were compared with those measured at the visit after diagnosis. A comparison of continuous variables between patient groups was performed using the Student t-test. Statistical comparison between different types of AF was performed using parametric (ANOVA) or nonparametric (Kruskal Wallis) tests appropriate for continuous or ordinal variables, while the chi-square test or Fisher exact test, as appropriate, was applied to discrete variables.
All comparisons performed were descriptive, and the null hypothesis was rejected in all cases with an alpha error less than 0.05. All statistical analyses were performed using the statistical package SAS® system version 9.1 for Windows 2000 or later versions, where available.
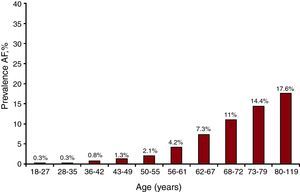
ResultsFor phase A of the study, 836 PC researchers saw a total of 119 526 patients (mean age 52.9 [15.2] years; 40.9% male) over 5 consecutive days. A total of 7260 had AF, yielding a frequency for this pathology of 6.1% (5.9-6.2, 95% confidence interval [95%CI]); AF was more common in men, 7.3% (95%CI, 7.1-7.5) vs 5.2% (95%CI, 5.1-5.4) in women. Furthermore, an age-related increase in AF was seen: 18-27 years, 0.27% (95%CI, 0.17-0.37); 28-35 years, 0.31% (95%CI, 0.21-0.41); 36-42 years, 0.79% (95%CI, 0.63-0.94); 43-49 years, 1.26% (95%CI, 1.07-1.46); 50-55 years, 2.12% (95%CI, 1.85-2.38); 56-61 years, 4.23% (95%CI, 3.86-4.59); 62-67 years, 7.3% (95%CI, 6.85-7.75); 68-72 years, 10.99% (95%CI, 10.39-11.59); 73-79 years, 14.39% (95%CI, 13.78-14.99); and 80-119 years, 17.56% (95%CI, 16.90 to 18.21), shown in Figure 1. AF was more common among hypertensive patients than in nonhypertensive ones (14% vs 1.9%, P<.001).
Figure 1. Presence of atrial fibrillation by age. AF, atrial fibrillation.
Phase B of the study included 3306 patients previously diagnosed with AF, of which 3287 were eligible for final data analysis. Table 1 summarizes the clinical characteristics of patients with AF. The high proportion of patients with cardiovascular risk factors was notable, mainly hypertension (92.6%), hypercholesterolemia (70.6%) and sedentarism (53.7%), associated vascular disease (mainly heart failure, 21.3%), and ischemic heart disease (20.9%). With regard to hypertension, 60.2% of patients were well controlled. More than half of patients with AF had left ventricular hypertrophy (55.3%). The diagnosis of left ventricular hypertrophy in PC was carried out mainly by electrocardiogram (79.6%) and less frequently by echocardiography (36%).
Table 1. Clinical Characteristics of Atrial Fibrillation Patients Treated in Primary Care.
| Biodemographic details | |
| Age, years | 71.9 (10.1) |
| Sex (male), % | 52.3 |
| Body mass index | 28.6 (4.5) |
| AF diagnosis time, years | 4.6 (4.1) |
| Cardiovascular risk factors | |
| Hypertension, % | 92.6 |
| Hypercholesterolemia, % | 70.6 |
| Hypertriglyceridemia, % | 38.6 |
| Diabetes mellitus, % | 33.7 |
| Metabolic syndrome, % | 32.6 |
| Early AF CVD, % | 14.5 |
| Smoking, % | |
| Smoker | 7.1 |
| Exsmoker <1 year | 5.9 |
| Exsmoker ≥1 year | 24.6 |
| Nonsmoker | 62.4 |
| Physical activity, % | |
| Sedentary | 53.7 |
| Moderate | 37.7 |
| Active | 8.6 |
| Vascular disease, % | |
| Heart failure | 21.3 |
| Ischemic heart disease | 20.9 |
| Valvular disease | 20.6 |
| Slight | 39.6 |
| Moderate | 43.3 |
| Severe | 17 |
| Kidney disease | 11.7 |
| Cerebrovascular disease | 11.1 |
| Peripheral artery disease | 9.6 |
| Advanced retinopathy | 2.9 |
| Physical examination | |
| Systolic blood pressure, mmHg | |
| Hypertensive | 137.5 (14.8) |
| Nonhypertensive | 125.9 (10.1) |
| Diastolic blood pressure, mmHg | |
| Hypertensive | 79.9 (9.5) |
| Nonhypertensive | 75.5 (7.3) |
| Heart rate | 77.2 (11.7) |
AF, atrial fibrillation; AF CVD, family history of cardiovascular disease.
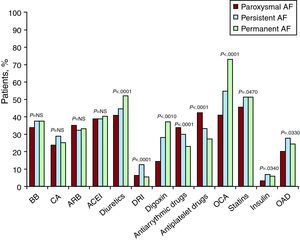
The different types of treatment, overall and by AF type, are shown in Figure 2. Diuretics, digoxin, and oral anticoagulants (OAC) were most frequently prescribed in patients with permanent AF, whereas antiplatelet and antiarrhythmic agents were prescribed for paroxysmal/persistent AF.
Figure 2. Current treatment by type of atrial fibrillation and for the overall sample. ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin receptor blockers; BB, beta blockers; CA, calcium antagonists; DRI, direct renin inhibitors; NS, non significant; OAD, oral antidiabetics; OCA, oral anticoagulation.
Table 2 shows data on the type of AF at diagnosis and its evolution over time. The 2 most common types of AF at the time of diagnosis were permanent AF (45.3%) and newly diagnosed AF (24.8%). Among patients newly diagnosed with AF, most evolved to permanent AF (57.4%), while 61.3% of those who had a diagnosis of paroxysmal AF remained with this diagnosis. Finally, about half of persistent AF patients developed permanent AF, with the other half continuing with persistent AF.
Table 2. Type of Atrial Fibrillation at Diagnosis and Evolution Over Time.
| Classification of AF at diagnosis, % | Evolution, % | ||
| New onset | 24.8 | Paroxysmal AF | 26 |
| Persistent AF | 16.6 | ||
| Permanent AF | 57.4 | ||
| Paroxysmal | 16.5 | Paroxysmal AF | 61.3 |
| Persistent AF | 9.1 | ||
| Permanent AF | 29.6 | ||
| Persistent | 13.4 | Paroxysmal AF | 1.3 |
| Persistent AF | 52.5 | ||
| Permanent AF | 46.1 | ||
| Permanent | 45.3 | Paroxysmal AF | 0.1 |
| Persistent AF | 1.1 | ||
| Permanent AF | 98.8 | ||
AF, atrial fibrillation.
The factors associated with progression to permanent AF, compared with the profile of patients who did not develop it, were older age (72.7 [10.0] years vs 69.1 [10.9] years, P<.0001), the presence of previous valvular disease (15.2% vs 10.1%, P<.01) and higher values of serum creatinine (1.01 [0.3] mg/dL vs 0.97 [0.3] mg/dL, P<.05). Among patients who progressed to permanent AF, a higher proportion of family history of cardiovascular disease was found (62.2% vs 48.6%, P<.0001), in particular, cerebrovascular disease (14.5% vs 7.9%, P<.0001), heart failure (21.9% vs 14.5%, P<.001), valvular disease (23.3% vs 14.2%, P<.0001), and kidney disease (12.6% vs 7.9%, P<.01). The serum creatinine values remained higher in the population of patients with permanent AF (1.03 [0.4] mg/dL vs 0.90 [0.3] mg/dL, P<.05).
The clinical characteristics of patients according to the presence of hypertension are shown in Table 3. Patients with high blood pressure generally had an increased prevalence of hypercholesterolemia, diabetes mellitus, metabolic syndrome, a sedentary lifestyle, and the presence of vascular disease (heart failure, ischemic heart disease, valvular heart disease, kidney disease, cerebrovascular disease, peripheral arterial disease, and advanced retinopathy); in those without hypertension, hypertriglyceridemia was more common. However, there were no differences with regard to smoking or a family history of premature cardiovascular disease.
Table 3. Clinical Characteristics of Patients According to the Presence of Hypertension.
| Without HPB | HPB | P | |
| Cardiovascular risk factors | |||
| Hypercholesterolemia, % | 66 | 71 | .0487 |
| Hypertriglyceridemia, % | 67.4 | 61 | .0171 |
| Diabetes mellitus, % | 10.4 | 35.6 | <.0001 |
| Metabolic syndrome, % | 14.6 | 34 | <.0010 |
| Early AF CVD, % | 15 | 14.5 | NS |
| Smoking, % | NS | ||
| Smoker | 7.5 | 7.1 | |
| Exsmoker <1 year | 7.1 | 5.9 | |
| Exsmoker ≥1 year | 24.7 | 24.6 | |
| Nonsmoker | 60.7 | 62.4 | |
| Physical activity, % | .0080 | ||
| Sedentary | 41.1 | 54.3 | |
| Moderate | 48.5 | 36.8 | |
| Active | 10.4 | 8.5 | |
| Vascular disease | |||
| Heart failure, % | 6.1 | 22.5 | <.0001 |
| Ischemic heart disease, % | 5.6 | 22 | <.0001 |
| Valvular disease, % | 12.8 | 21.1 | <.0001 |
| Kidney disease, % | 4.1 | 12.3 | <.0001 |
| Cerebrovascular disease, % | 5.1 | 11.5 | <.0001 |
| Peripheral artery disease, % | 5.1 | 10 | <.0001 |
| Advanced retinopathy, % | 0.5 | 3 | <.0001 |
AF CVD, family history of cardiovascular disease; HPB: high blood pressure; NS, non significant.
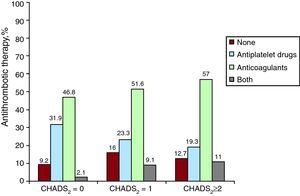
For thromboembolic risk, 4.5% of patients with AF had a CHADS2 score of 0, 28.1% of patients scored 1 and 67.4% scored ≥2 (Table 4). OAC were prescribed for 46.8% of patients with CHADS2=0, and 51.6% of patients with CHADS2 ≥1, with 23.3% of the latter also taking oral antiplatelet agents. Of all patients with CHADS2 ≥2, 57.0% took OAC and 19.3% antiplatelet agents (Figure 3).
Table 4. Percentage of Patients With Different CHADS2 Scale Components.
| CHADS2 Scale Variables | % Patients | CHADS2 Score | % Patients |
| Hypertension | 92.4 | 0 | 4.5 |
| Age ≥75 years | 44.7 | 1 | 28.1 |
| Diabetes mellitus | 33.7 | 2 | 31.6 |
| Congestive heart failure | 22 | 3 | 21 |
| Previous stroke episode | 13.9 | >3 | 14.8 |
Figure 3. Antithrombotic therapy according to the CHADS2 score.
DiscussionOne of the main objectives of this study was to determine the prevalence of AF in PC. In the largest sample studied to date in Spain (almost 120 000 subjects, with a mean age of 52.9 years), the proportion of patients seen in PC with AF was 6%. There have been other studies of a smaller sample size in Spain estimating the prevalence of AF in patients seen in PC. For example, the CARDIOTENS study (mean age 68.4 years) reported 2.75% of subjects with AF in PC (vs 17.62% in specialist care); the BARBANZA study (mean age 54 years) reported 4%; the PREV-ICTUS study (mean age 71.9 years) reported 10.2%; and the FAPRES study (age 72.8 years) gave 10.3%.7, 10, 11, 13 In other words, the presence of AF in PC is high.14 Moreover, as expected, the prevalence of AF increased with age, very significantly after the age of 60; it exceeded 7% in patients aged 62 years, and reached almost 18% in those over 80. The mean age was 53 years, and the number of subjects with AF is greater in older populations. In light of these data, it seems clear that the prevalence of AF in Spain will increase in the coming years due to the progressive aging of the population, with the consequent increase in associated costs.15 Interestingly, almost all patients with AF seen in PC had a history of hypertension (92%), compared to 66% of patients diagnosed with permanent AF in the CARDIOTENS study; the percentage observed in the PREV-ICTUS study (92.1%) was very similar to our results.7, 11 Furthermore, a high percentage of patients with other cardiovascular risk factors and associated cardiovascular disease was observed, mainly heart failure (21%) and ischemic heart disease (21%). Along with the common presence of cardiovascular disease in AF patients, other studies have shown that the presence of AF is common in patients with heart failure and ischemic heart disease, indicating a close relationship between AF and these conditions.16, 17, 18
One of the most interesting and novel aspects of this study was to assess the clinical profile of patients who progressed to permanent AF in comparison with the profile of patients who did not. Factors associated with the development of permanent AF were older age, the presence of valvular heart disease, and cardiovascular or renal disease. In a Canadian study conducted in subjects with paroxysmal AF, it was found that factors associated with an increased risk of progression to permanent AF were increasing age, significant valvular disease (significant aortic stenosis or mitral insufficiency), enlarged left atrium, and the diagnosis of cardiomyopathy. On the other hand, as in this study, a higher heart rate during AF reduced the risk of progression. This is probably because it was more symptomatic, and treatment was started earlier than in the cases of paucisymptomatic or asymptomatic AF.19 These data indicate that to reduce the risk of permanent AF it is probably important to aggressively treat the different risk factors and cardiovascular disease associated with AF from the initial stages, to try to prevent the development of a substrate leading to the presence of AF.
There is a very close relationship between hypertension and AF. Hypertension produces a number of structural and electrophysiological changes in the heart that lead to the development of AF.20 In our study, patients with hypertension and AF had more comorbidity than did patients without hypertension. This could partly explain the high risk of complications in subjects with both conditions.21 For this reason, there is particular interest in preventing the development of AF in hypertensive patients. Although the most important factor here is to achieve adequate blood pressure control, it has been suggested that inhibition of the renin-angiotensin system may provide additional benefits in patients with hypertension and left ventricular hypertrophy or in those with heart failure.22, 23, 24 Interestingly, those patients treated with direct renin inhibitors had fewer cases of permanent AF. For example, aliskiren may reduce the risk of paroxysmal or persistent AF becoming permanent. However, since this is a cross-sectional study with the limitations inherent to this type of registries, this potential can only be confirmed by a larger study designed specifically for this purpose.
As regards the thromboembolic risk, just over two-thirds of patients had a CHADS2 score ≥2, so most AF patients seen in PC have a clear indication for OAC. However, almost one-third of patients with CHADS2 ≥2 were not receiving anticoagulants. Numerous studies indicate that there is an underuse of OAC in patients with AF.25, 26 However, this study also found the opposite: nearly half of patients with a CHADS2=0 were being treated with OAC when, according to the guidelines, there is no indication for anticoagulant treatment. There were also significant differences in the use of OAC according to the type of AF, as patients with permanent AF were more often prescribed OAC than patients with paroxysmal or persistent AF even though OAC indication should be independent of the type of AF.1, 9 It therefore seems necessary to improve the training of PC physicians regarding appropriate OAC indications in AF patients.
This was an observational, cross-sectional study, sufficient to establish both the frequency of AF and the clinical profile of AF patients seen in PC. However, it did not provide for prospective follow-up of patients and allowed only hypothesis generation about how a previous diagnosis could influence the occurrence of certain circumstances, such as the factors that may lead to the development of permanent AF. Prediagnosis data were based on patient history. While this may involve certain limitations, the researchers were urged to collect the data thoroughly, based on comprehensive clinical reports. Also, it was stressed that patients were to be recruited consecutively, but this instruction may not have been strictly followed. However, given the very high number of subjects, we believe that any breach of strict consecutive sampling would most likely not be significant. Since the study was conducted throughout the country, the results are applicable to subjects treated in PC in Spain, but not necessarily in other populations with different health systems or different clinical profiles.
ConclusionsAtrial fibrillation is present in 6.1% of PC patients. This percentage increases with age, male sex, and hypertension. Patients with AF have high comorbidity with numerous risk factors and associated cardiovascular diseases. The most common type of AF is permanent AF. Age and cardiac and renal diseases are associated with the development of permanent AF. Anticoagulant therapy in AF patients treated in PC is far from optimal. Although more than two-thirds of patients had a CHADS2 score ≥2, almost one-third of them were not being treated with anticoagulants. Moreover, almost half of patients with a low risk of stroke (CHADS2=0) were taking OAC even though such treatment is not indicated.
FundingThe study was funded in a nonrestrictive manner by the pharmaceutical company Novartis Farmacéutica, SA. The funder did not intervene in any way in the organization, management or analysis of the data.
Conflicts of interestNone declared.
Acknowledgements
The authors wish to express their deepest and most sincere gratitude to the physicians who participated with enthusiasm and dedication in the VAL-FAAP study, and to the members of the Primary Care Working Group in the Clinical Cardiology Section: Francisco Javier Alonso Moreno (Toledo); Carlos Brotons Cuixart (Barcelona); Alberto Calderón Montero (Madrid); Mariano de la Figuera von Wichmann (Barcelona); Santiago Díaz Sánchez (Madrid); Josep Franch Nadal (Barcelona); José Luis Llisterri Caro (Valencia); José María Lobos Bejarano (Madrid); Iñaki Mabe Angulo (Bilbao); Javier Mediavilla Bravo (Burgos); Gustavo Rodríguez Roca (Toledo); Manuel Taboada Taboada (Madrid); Gonzalo Barón Esquivias (Seville); Vivencio Barrios Alonso (Madrid); Xavier Borrás Pérez (Barcelona); Ramón Bover Freire (Madrid); Carlos Escobar Cervantes (Madrid); Juan José Gómez Doblas (Málaga); Nekane Murga Eizagaetxebarria (Bilbao).
Received 30 June 2011
Accepted 11 August 2011
Corresponding author: Servicio de Cardiología, Hospital Ramón y Cajal, Ctra. de Colmenar Viejo, Km 9.100, 28034 Madrid, Spain. vbarriosa@meditex.es