Heart failure with preserved ejection fraction (HFpEF) is a highly prevalent syndrome with an elevated risk of morbidity and mortality. To date, there is scarce evidence on the role of peak exercise oxygen uptake (peak VO2) for predicting the morbidity burden in HFpEF. We sought to evaluate the association between peak VO2 and the risk of recurrent hospitalizations in patients with HFpEF.
MethodsA total of 74 stable symptomatic patients with HFpEF underwent a cardiopulmonary exercise test between June 2012 and May 2016. A negative binomial regression method was used to determine the association between the percentage of predicted peak VO2 (pp-peak VO2) and recurrent hospitalizations. Risk estimates are reported as incidence rate ratios.
ResultsThe mean age was 72.5 ± 9.1 years, 53% were women, and all patients were in New York Heart Association functional class II to III. Mean peak VO2 and median pp-peak VO2 were 10 ± 2.8mL/min/kg and 60% (range, 47-67), respectively. During a median follow-up of 276 days [interquartile range, 153-1231], 84 all-cause hospitalizations in 31 patients (41.9%) were registered. A total of 15 (20.3%) deaths were also recorded. On multivariate analysis, accounting for mortality as a terminal event, pp-peak VO2 was independently and linearly associated with the risk of recurrent admission. Thus, and modeled as continuous, a 10% decrease of pp-peak VO2 increased the risk of recurrent hospitalizations by 32% (IRR, 1.32; 95%CI, 1.03-1.68; P = .028).
ConclusionsIn symptomatic elderly patients with HFpEF, pp-peak VO2 predicts all-cause recurrent admission.
Keywords
Heart failure (HF) is a major public health problem with high associated morbidity and mortality worldwide.1 More than 50% of patients with HF have preserved left ventricular ejection fraction (HFpEF), which is especially common in elderly people, women, and highly comorbid patients.2–4 Risk prediction of repeat hospitalizations in HF has often been overlooked and most of the studies endorsing risk prediction of hospitalizations have focused on time-to-first event analysis, ignoring the clinical impact of recurrent hospitalization that frequently occurs in HF.5 Recent initiatives advocate the inclusion of recurrent hospitalizations in risk stratification6 to transmit a more realistic picture of the disease burden.
Although the clinical value of peak exercise oxygen uptake (peak VO2) in HF with reduced ejection fraction is well-documented,7,8 the evidence endorsing its prognostic role in HFpEF is scarce7,9–11 and even absent, especially regarding the risk of recurrent admissions.
The purpose of this study was to evaluate whether the percentage of predicted peak VO2 (pp-peak VO2) is associated with the risk of recurrent admissions in elderly patients with HFpEF.
METHODSStudy Design and PatientsThis was a prospective study that included patients with a diagnosis of HFpEF according to the criteria of the European Society of Cardiology12 and New York Heart Association functional class II-III/IV between 2 periods: June 2012 to May 2013 and June 2015 to May 2016. The study was conducted in a single third-level center in Spain. All patients provided signed informed consent before participation. The protocol was approved by the research ethics committee of our center in accordance with the principles of the Declaration of Helsinki and national regulations.
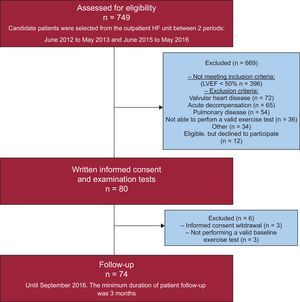
Candidate patients were selected from the outpatient HF unit.13 All patients met the following inclusion criteria: a) previous history of symptomatic HF (New York Heart Association functional class ≥ II); b) normal left ventricular ejection fraction (ejection fraction > 0.50 by the Simpson method and end-diastolic diameter < 60mm); c) structural heart disease (left ventricle hypertrophy/left atrial enlargement) and/or diastolic dysfunction estimated by 2-dimensional echocardiography; d) previous admission for acute HF; and e) clinical stability, without hospital admissions in the past 3 months. Patients were excluded if they could not perform a valid baseline exercise test or showed any previous medical condition such as: unstable angina, myocardial infarction or cardiac surgery within the previous 3 months; chronic metabolic, orthopedic, infectious disease or pulmonary disease (including pulmonary arterial hypertension, chronic thromboembolic pulmonary disease or chronic obstructive pulmonary disease); steroid, hormone, or cancer therapy; acute HF decompensation; any other comorbidity with a life expectancy of less than 1 year. The patient flowchart is depicted in Figure 1.
After signing the informed consent form, a comprehensive medical history, physical examination, anthropometry and examination tests were performed by 2 trained cardiologists.
ProceduresStudy procedures included electrocardiogm, echocardiography, cardiopulmonary exercise testing, and blood samples for a panel of baseline biomarkers. All of them were performed on the same day.
Cardiopulmonary Exercise TestingMaximal functional capacity was evaluated with an incremental and symptom-limited cardiopulmonary exercise test (CORTEX Metamax 3B) on a bicycle ergometer, beginning with a workload of 10W and increasing stepwise at 10W increments every 1minute. During exercise, patients were continuously monitored with a 12-lead electrocardiogram and blood pressure measurements every 2minutes. Gas exchange data and cardiopulmonary variables were averaged every 10seconds. Peak VO2 was considered the highest value of VO2 during the last 20seconds of exercise and pp-peak VO2 was calculated using the Wasserman equation.14 Exercise ventilatory efficiency (VE/VCO2 slope) was determined by measuring the slope across the entire course of the exercise.15
EchocardiographyDoppler echocardiogram examinations were performed under resting conditions using 2-dimensional echocardiography (iE33, Philips). All parameters, including tissue Doppler parameters, were measured according to the current guidelines of the European Society of Echocardiography.16
BiomarkersAll blood samples were obtained between 09:00 a.m. and 12:00 p.m. All biomarkers were measured using established commercial essays.
Endpoints and Follow-upThe total number of all-cause unplanned hospitalizations was selected as the primary endpoint. Cardiovascular admissions were selected as the secondary endpoint. Cardiovascular admissions were considered as those occurring due to acute HF, acute coronary syndrome, arrhythmias, stroke, or other cardiovascular causes, such as rupture of an aneurysm, peripheral ischemia, or aortic dissection. Hospitalizations were identified from the clinical records of patients in the HF unit and hospital wards and from electronic medical records. Fatal events were identified from the clinical records of the HF unit, hospital wards, emergency room, and general practitioners and by contacting the patient's relatives. All patients included were foowed up until September 2016. The minimum duration of patient follow-up was 3 months.
Statistical AnalysisContinuous and categorical variables are presented as the mean ± standard deviation, median [interquartile range] or percentages, as appropriate. Crude rates (number of events per 10 person-year) are presented for pp-peak VO2 quartiles (pp-peak VO2Q). Bivariate negative binomial regression was used to assess the independent association between pp-peak VO2 with the endpoint.17 This method simultaneously models the number of admissions with the mortality event and uses a shared frailty to account for the correlation between these 2 outcomes. Thus, the estimates for recurrent admissions are internally adjusted by mortality as a terminal event (informative dropout). To account for differences in the observation time per patient, we used the natural logarithm of the observation time to offset the model for admission endpoints as well as for mortality. Estimates are reported as incidence rate ratio (IRR). All variables listed in Table 1 were evaluated for prognostic purposes. A backward stepwise selection, with the Akaike information criterion as the stopping criterion, was used to achieve a parsimonious model. The linearity assumption for continuous variables was simultaneously tested and transformed, if appropriate, with fractional polynomials. The pp-peak VO2 was modeled using a fractional polynomial transformation of 0 (natural logarithm), per decrease in 10%, and as pp-peak VO2Q. The covariates included in the final models for all-cause and cardiovascular rehospitalization are included in the legend of Figure 2. A 2-sided P-value of < .05 was considered to be statistically significant. All analyses were performed using Stata 14.0.
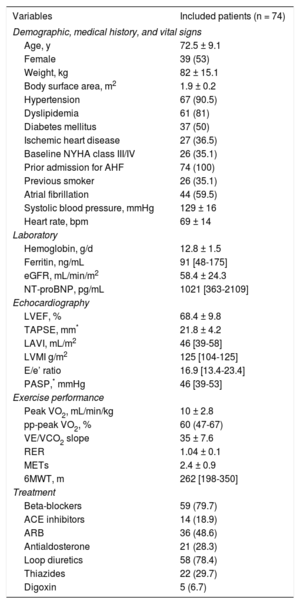
Baseline Characteristics of the Study Population
| Variables | Included patients (n = 74) |
|---|---|
| Demographic, medical history, and vital signs | |
| Age, y | 72.5 ± 9.1 |
| Female | 39 (53) |
| Weight, kg | 82 ± 15.1 |
| Body surface area, m2 | 1.9 ± 0.2 |
| Hypertension | 67 (90.5) |
| Dyslipidemia | 61 (81) |
| Diabetes mellitus | 37 (50) |
| Ischemic heart disease | 27 (36.5) |
| Baseline NYHA class III/IV | 26 (35.1) |
| Prior admission for AHF | 74 (100) |
| Previous smoker | 26 (35.1) |
| Atrial fibrillation | 44 (59.5) |
| Systolic blood pressure, mmHg | 129 ± 16 |
| Heart rate, bpm | 69 ± 14 |
| Laboratory | |
| Hemoglobin, g/d | 12.8 ± 1.5 |
| Ferritin, ng/mL | 91 [48-175] |
| eGFR, mL/min/m2 | 58.4 ± 24.3 |
| NT-proBNP, pg/mL | 1021 [363-2109] |
| Echocardiography | |
| LVEF, % | 68.4 ± 9.8 |
| TAPSE, mm* | 21.8 ± 4.2 |
| LAVI, mL/m2 | 46 [39-58] |
| LVMI g/m2 | 125 [104-125] |
| E/e’ ratio | 16.9 [13.4-23.4] |
| PASP,* mmHg | 46 [39-53] |
| Exercise performance | |
| Peak VO2, mL/min/kg | 10 ± 2.8 |
| pp-peak VO2, % | 60 (47-67) |
| VE/VCO2 slope | 35 ± 7.6 |
| RER | 1.04 ± 0.1 |
| METs | 2.4 ± 0.9 |
| 6MWT, m | 262 [198-350] |
| Treatment | |
| Beta-blockers | 59 (79.7) |
| ACE inhibitors | 14 (18.9) |
| ARB | 36 (48.6) |
| Antialdosterone | 21 (28.3) |
| Loop diuretics | 58 (78.4) |
| Thiazides | 22 (29.7) |
| Digoxin | 5 (6.7) |
6MWT, 6-minute walk test; ACE, angiotensin-converting enzyme; AHF, acute heart failure; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate using the Modification of Diet in Renal Disease formula; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; METs, metabolic equivalents; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; peak VO2, peak exercise oxygen uptake; pp-peak VO2, percentage of predicted peak exercise oxygen uptake; RER, respiratory exchange ratio; TAPSE, tricuspid annular plane systolic excursion; VE/VC02 slope, relationship between minute ventilation and the rate of CO2 elimination;
Continuous and categorical variables are presented as mean ± standard deviation, median [interquartile range] or No. (%), as appropriate.
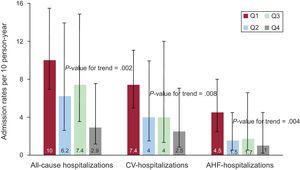
Rates of all-cause, CV and AHF hospitalizations according to quartiles of percentage of predicted peak exercise oxygen uptake. AHF, acute heart failure; CV, cardiovascular.
Q1 = 26.1% to 46.4% of predicted peak exercise oxygen uptake.
Q2 = 47% to 60% of predicted peak exercise oxygen uptake.
Q3 = 60% to 67.5% of predicted peak exercise oxygen uptake.
Q4 = 67.7% to 102.8% of predicted peak exercise oxygen uptake.
The mean ± standard deviation of the patients’ age was 72.5 ± 9.1 years, 53% were female, 35.1% were in New York Heart Association functional class III, and the median [interquartile range] for N-terminal pro-B-type natriuretic peptide was 1022 pg/mL [363-2109]. The mean ± standard deviation and median [interquartile range] for peak VO2 and pp-peak VO2 were 10 ± 2.8mL/min/kg and 60 [47-67], respectively. The remaining baseline characteristics of the sample are summarized in Table 1. There was no serious adverse event during cardiopulmonary exercise testing.
The patients were followed up for a median of 276 days [interquartile range, 153-1231]. During the follow-up, 15 deaths (20.3%) and 84 all-cause hospitalizations in 31 patients (41.9%) were registered, distributed as follows: 1 hospitalization (n = 11 [14.9%]), 2 hospitalizations (n = 9 [12.2%]), 3 hospitalizations (n = 4 [5.4%]), 4 hospitalizations (n = 3 [4.1%]), 5 hospitalization (n = 5 [6.8%]), and 6 hospitalizations (n = 1 [1.4%]). Most rehospitalizations were due to cardiovascular causes (n = 62 hospitalizations [73.8% of all-causes]) including acute HF as the most frequent among cardiovascular causes (n = 33 hospitalizations [39.3% of all-causes]). The crude rehospitalization rates across pp-peak VO2Q showed a significant and stepwise increase when moving from higher to lower quartiles (P < .01): 2.9, 7.4, 6.2, and 10 hospitalizations per 10 person-year for Q4 (67.7%-102.8%), Q3 (60%-67.5%), Q2 (47%-60%) and Q1 (26.1%-46.4%), respectively. Likewise, increased rates of cardiovascular and acute HF admissions were found among the lower quartiles of pp-peak VO2Q (Figure 2).
Percentage of Predicted Peak Exercise Oxygen Uptake and Risk of All-cause Recurrent AdmissionsIn a univariate setting, pp-peak VO2 was significantly and inversely associated with the risk of recurrent all-cause admissions. Thus, risk estimates attributable to pp-peak VO2 evaluated as continuous (per decrease in 10%) and as a natural logarithm (per decrease in 1 log) were (IRR, 1.30; 95% confidence interval [95%CI], 1.06-1.61; P = .014 and IRR, 3.94; 95%CI, 1.40-11.05; P = .009), respectively.
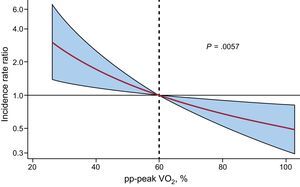
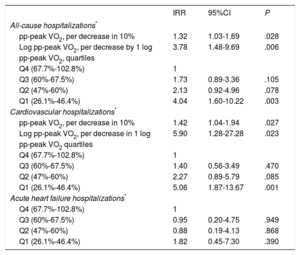
In a multivariate scenario, including well-established prognosticators and potential confounders (age, atrial fibrillation, systolic blood pressure, heart rate, E/e’ ratio, hemoglobin and N-terminal pro-B-type natriuretic peptide) in the risk model, the inverse association between pp-peak VO2 and the risk of repeat hospitalizations remained significant (Table 2). The risk gradient showed an inverse and almost linear relationship between pp-peak VO2 and the risk of all-cause readmissions (Figure 3). When pp-peak VO2 was modeled as continuous, a 10% decrease of pp-peak VO2 increased the risk of recurrent hospitalizations by 32% (IRR, 1.32; 95%CI, 1.03-1.68; P = .028) (Table 2). In a sensitivity analysis, forcing in the multivariate analysis other important cardiopulmonary exercise prognosticators such as the VE/VCO2 slope, ppVO2 (per decrease of 10%) remained significantly associated with this endpoint (IRR, 1.31; 95%CI, 1.05-1.65; P = .020) and the VE/VCO2 slope was borderline associated with the risk of recurrent hospitalizations (IRR, 1.03; 95%CI, 0.99-1.06; P = .088).
Risk Estimates of All-cause Hospitalizations and Cardiovascular Hospitalizations
| IRR | 95%CI | P | |
|---|---|---|---|
| All-cause hospitalizations* | |||
| pp-peak VO2, per decrease in 10% | 1.32 | 1.03-1.69 | .028 |
| Log pp-peak VO2, per decrease by 1 log | 3.78 | 1.48-9.69 | .006 |
| pp-peak VO2, quartiles | |||
| Q4 (67.7%-102.8%) | 1 | ||
| Q3 (60%-67.5%) | 1.73 | 0.89-3.36 | .105 |
| Q2 (47%-60%) | 2.13 | 0.92-4.96 | .078 |
| Q1 (26.1%-46.4%) | 4.04 | 1.60-10.22 | .003 |
| Cardiovascular hospitalizations* | |||
| pp-peak VO2, per decrease in 10% | 1.42 | 1.04-1.94 | .027 |
| Log pp-peak VO2, per decrease in 1 log | 5.90 | 1.28-27.28 | .023 |
| pp-peak VO2 quartiles | |||
| Q4 (67.7%-102.8%) | 1 | ||
| Q3 (60%-67.5%) | 1.40 | 0.56-3.49 | .470 |
| Q2 (47%-60%) | 2.27 | 0.89-5.79 | .085 |
| Q1 (26.1%-46.4%) | 5.06 | 1.87-13.67 | .001 |
| Acute heart failure hospitalizations* | |||
| Q4 (67.7%-102.8%) | 1 | ||
| Q3 (60%-67.5%) | 0.95 | 0.20-4.75 | .949 |
| Q2 (47%-60%) | 0.88 | 0.19-4.13 | .868 |
| Q1 (26.1%-46.4%) | 1.82 | 0.45-7.30 | .390 |
95%CI, 95% confidence interval; hospitalizations; IRR, incidence rate ratio; Log pp-peak VO2, logarithm of percentage of predicted peak exercise oxygen uptake; NT-proBNP, N-terminal pro-B-type natriuretic peptide; pp-peak VO2, percentage of predicted peak exercise oxygen uptake.
Risk estimates are reported as IRR.
Relationship between pp-peak VO2 and the risk of all-cause readmission after multivariate adjustment. pp-peak VO2, percentage of predicted peak exercise oxygen uptake. Adjusted for age, atrial fibrillation, systolic blood pressure, heart rate, E/e’ ratio, hemoglobin and N-terminal pro-B-type natriuretic peptide.
Univariate estimates showed an inverse relationship between pp-peak VO2 and cardiovascular admissions. The pp-peak VO2 (per 10% decrease) and logarithm of pp-peak VO2 (per decrease by 1 log) were inversely associated with a higher risk of cardiovascular hospitalizations (IRR, 1.28; 95%CI, 0.99-1.66; P = .063 and IRR, 3.73; 95%CI, 1.05-13.24; P = .042, respectively). After a multivariate adjustment, this inverse association was strengthened (Table 2). Regarding acute HF hospitalizations, multivariate analysis showed that pp-peak VO2 (per 10% decrease) and the logarithm of pp-peak VO2 (per decrease by 1 log) were not significantly related to the risk of repeat acute HF admissions (IRR, 1.10; 95%CI, 0.25-4.73; P = .583 and IRR, 1.55; 95%CI, 0.36-6.69; P = .556, respectively). Similarly, quartiles of pp-peak VO2 were not related to the risk of repeat acute HF hospitalizations (Table 2).
DISCUSSIONEpidemiological studies have shown that the prevalence of HFpEF compared with HF with reduced ejection fraction is increasing over time; however, the prognosis and optimal pharmacological armamentarium for HFpEF is still somber.4,12 The main finding of our study was that pp-peak VO2 was independently and linearly associated with recurrent admissions in a cohort of elderly persons with highly symptomatic HFpEF. To our knowledge, this is the first study that has evaluated the prognostic usefulness of pp-peak VO2 for predicting recurrent hospitalizations in this type of patient.
The evidence available to date has shown that patients with HFpEF have markedly reduced functional capacity as objectively measured by peak VO218 but little is known about the relationship between pp-peak VO2 and prognosis in HFpEF patients. To date, only 3 studies have evaluated the prognostic value (time-to-first cardiac-related mortality or hospitalization) of peak VO2 and other cardiopulmonary exercise testing parameters in patients with HF and diastolic dysfunction.9–11 In 2005, Guazzi et al.11 studied 46 young male (mean age 57.9 ± 13 years) HF patients with diastolic dysfunction and a left ventricular ejection fraction ≥ 50%. These authors found that both the peak VO2 and the VE/VCO2 slope were significant predictors of mortality and rehospitalization on univariate analysis; however, after multivariate adjustment, only the VE/VCO2 slope remained independently related to adverse prognosis. In 2011, Yan et al.10 evaluated 224 predominately male (71%) HFpEF patients with a mean age of 68.8 ± 9 years. Similarly, these authors found that plasma B-type natriuretic peptide and VE/VCO2 slope, but not peak VO2, had independent and incremental prognostic value for all-cause and cardiovascular mortality. More recently, in a retrospective study of 173 young and predominately black male HF patients with a left ventricular ejection fraction ≥ 50%, Shafiq et al.9 reported that peak VO2 and pp-peak VO2 had a strong prognostic role in terms of predicting the composite of all-cause mortality and/or cardiac transplant.
In our opinion, previous and current studies endorse the usefulness of cardiopulmonary exercise testing for risk stratification of patients with HFpEF. Discrepancies about the most accurate parameter for risk stratification may be attributed to the small sample size of the studies, differences in baseline characteristics, the covariates included in the multivariate adjustments, and the methodological approach for evaluating the clinical endpoints (recurrent events vs time-to-first event). The main strength of the present study lies in the clinical characteristics of the patients included (elderly, highly comorbid patients with important functional impairment19). Last, in our view, analyzing the risk of recurrent hospitalizations in contrast to time-to-first hospitalizations approaches, adds consistency and validity to our findings.
We believe the present findings endorse the clinical value of peak VO2 over other traditional, but more subjective, parameters of disease severity such as New York Heart Association functional classification.20 Furthermore, peak VO2 emerges as a reliable and accurate surrogate endpoint to evaluate new therapeutic strategies in HFpEF. For instance, our group recently found a significant increase of peak VO2 after a home-based program of inspiratory muscle training in a small study of patients with HFpEF.21
LimitationsThis study has some limitations. First, this is a single center observational study in which there may have been many potential confounders; second, the small number of adverse events registered, such as mortality and acute HF hospitalizations, precluded evaluation of the independent association between pp-peak VO2 and those events; third, these findings cannot be directly extrapolated to patients with milder forms of the disease; and fourth, the use of a bicycle exercise protocol rather than treadmill exercise testing could have led to underestimation of functional capacity in some patients.14
CONCLUSIONSIn symptomatic elderly patients with HFpEF, pp-peakVO was significantly and inversely associated with the risk of long-term repeat hospitalizations. Further studies are needed to confirm these results.
FUNDINGThis work was supported in part by grants from: Sociedad Española de Cardiología: Investigación Clínica en Cardiología, Grant SEC 2015, CIBER CV 16/11/00420, 16/11/00403, FEDER and PIE15/00013.
CONFLICTS OF INTERESTNone declared.
- –
Heart failure with preserved ejection fraction is the most prevalent form of HF.
- –
Heart failure with preserved ejection fraction is especially common in the aging population, women, frail persons, and highly comorbid patients.
- –
The clinical value of peak VO2 in HFpEF is not well-documented.
- –
The evidence endorsing the prognostic role of peak VO2 in HFpEF is scarce and even absent, especially regarding the risk of recurrent admissions.
- –
The main finding of our study was that pp-peak VO2 was independently and linearly associated with recurrent admissions in a cohort of elderly persons and highly symptomatic HFpEF patients.
- –
To our knowledge, this is the first study that has evaluated the prognostic usefulness of pp-peak VO2 for predicting recurrent hospitalizations in this type of patient.