.
Nonvalvular atrial fibrillation (NVAF) is the most frequently occurring arrhythmia in Spain. Incidence is closely related to age, with prevalence close to 25% in patients older than 80 years.1 Among the many processes associated with NVAF, cerebrovascular accident of cardioembolic origin is probably the most serious complication as it leads to high rates of disability and mortality.2 Another problem associated with atrial fibrillation is the need for oral anticoagulation (OAC) therapy to prevent cardioembolic events. This need grows with advanced age as the risk of an event is even greater.1 Moreover, the risk of complications associated with anticoagulation therapy, especially of bleeding, also increases in these patients.3 Currently, percutaneous left atrial appendage (LAA) occlusion is an alternative for patients with NVAF and contraindications to OAC treatment.4,5
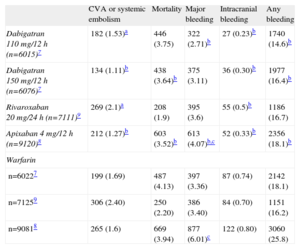
ORAL ANTICOAGULATION THERAPY: EFFICACY AND LIMITATIONSAnticoagulation therapy with vitamin K antagonists is considered the standard treatment for NVAF. The principal problems with these drugs are the increased bleeding risk, need for regular check-ups, interaction with food or other drugs, and instability of drug action in some cases. It is estimated that between 30% and 50% of patients indicated for OAC do not receive them.6 With the introduction of new anticoagulants such as dabigatran,7 apixaban,8 and rivaroxaban,9 management of these patients could change. Whatever the case may be, and despite their more stable and safer action profile, the bleeding risk, with an annual rate of between 2.1% and 3.6%, continues to be the principal Achilles’ heel of these new agents (Table). In fact, more recent registries indicate that incidence of cardioembolic events and hemorrhage secondary to dabigatran could be similar to those of warfarin.10 Despite the introduction of new anticoagulant agents, the percentage of patients indicated for OAC but not receiving treatment remains around 40%.11 Faced with poor adherence to therapy and the persistent risk of hemorrhage, the need for alternatives to anticoagulation therapy has become a priority for these patients.
Efficacy and Safety of the New Anticoagulation Therapies vs Warfarin
| CVA or systemic embolism | Mortality | Major bleeding | Intracranial bleeding | Any bleeding | |
| Dabigatran 110 mg/12 h (n=6015)7 | 182 (1.53)a | 446 (3.75) | 322 (2.71)b | 27 (0.23)b | 1740 (14.6)b |
| Dabigatran 150 mg/12 h (n=6076)7 | 134 (1.11)b | 438 (3.64)b | 375 (3.11) | 36 (0.30)b | 1977 (16.4)b |
| Rivaroxaban 20 mg/24 h (n=7111)9 | 269 (2.1)a | 208 (1.9) | 395 (3.6) | 55 (0.5)b | 1186 (16.7) |
| Apixaban 4 mg/12 h (n=9120)8 | 212 (1.27)b | 603 (3.52)b | 613 (4.07)b,c | 52 (0.33)b | 2356 (18.1)b |
| Warfarin | |||||
| n=60227 | 199 (1.69) | 487 (4.13) | 397 (3.36) | 87 (0.74) | 2142 (18.1) |
| n=71259 | 306 (2.40) | 250 (2.20) | 386 (3.40) | 84 (0.70) | 1151 (16.2) |
| n=90818 | 265 (1.6) | 669 (3.94) | 877 (6.01)c | 122 (0.80) | 3060 (25.8) |
CVA, cerebrovascular accident.
The figures express no. (% annual).
The LAA is an embryological remnant and its principal function is to control blood volume. It is located very near the left circumflex artery, bordering at the upper level with the upper left pulmonary vein and at the lower level with the mitral valve. LAA morphology is extraordinarily heterogeneous from one patient to another and there is often more than one lobe. In sinus rhythm, the LAA is a contractile structure that empties its content at each heartbeat. In atrial fibrillation, the LAA loses its contractile capacity and dilates, leading to a slowing of the blood flow, with the consequent increased risk of thrombosis. In pathology studies of patients with NVAF, 91% of thrombi located in the left atrium are found in the LAA. This has led to the belief that percutaneous LAA occlusion might be an effective strategy to prevent cardioembolic risk in patients with NVAF.
Percutaneous LAA occlusion is still in its initial stage and some years still must pass before we can gain a more reliable view of its role in patients with NVAF. Even if, in the future, it might constitute a real alternative for patients with no contraindications for OAC, currently its use should be reserved to those patients with contraindications to anticoagulation therapy, as recommended in European guidelines (IIb indication).5 In fact, most procedures in patients receiving anticoagulation therapy are referred either by the neurology service after an episode of intracranial bleeding, or by gastroenterology following observation of recurrent digestive bleeding with no treatable cause. The lack of valid alternatives to OAC to prevent cardioembolic risk, which is generally high as these are typically older patients with multiple pathologies, make LAA occlusion a highly attractive option for these patients. Labile international normalized ratio or the appearance of a cardioembolic event despite OAC treatment and adequate international normalized ratio are other contexts in which percutaneous LAA occlusion could be justified. In any case, multidisciplinary, individualized decisions are needed to assess both cardioembolic and bleeding risk and other essential factors like the effectiveness of treatment, frailty of the patient, or adherence to treatment, especially with OAC.
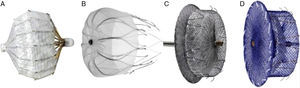
PERCUTANEOUS LEFT ATRIAL APPENDAGE OCCLUSION DEVICESThe PLAATO™ device was the first percutaneous LAA occluder (Fig. 1). Despite good preliminary results in terms of efficacy and safety, the development program was suspended and the device disappeared from the market.
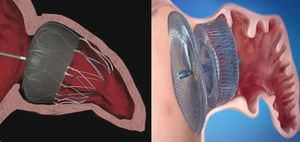
Currently, the two devices most frequently used worldwide are the Watchman™ system (Boston Scientific; Boston, Massachusetts, United States) and the Amplatzer™ Cardiac Plug (ACP) (St. Jude Medical; Minneapolis, Minnesota, United States) (Fig. 1). Like the PLAATO™ system, both are implanted via transseptal pathway using femoral vein access. Both prostheses are highly flexible and have a system of stabilizing guidewires that anchor to the LAA wall and thus avoid embolization. The principal difference between the two devices, however, is in their shape. The Watchman™ system is implanted at 10 mm from the LAA ostium, and therefore does not cover it; the ACP contains a lobe that is implanted 10-15 mm from the ostium and a disc that completely covers the LAA ostium (Fig. 2). A second generation ACP called the Amplatzer™ Amulet™ has recently appeared on the market. The Amulet™ device (Fig. 1) is also lobe-shaped and has a disc like the ACP, but has modifications that facilitate device preparation and implantation and, at the same time, minimize the risk of embolization and thrombosis.
Position of the Watchman™ and Amplatzer™ Cardiac Plug devices after implantation in the left atrial appendage. Watchman™ device implanted at 10 mm from the ostium of the left atrial appendage (left) and Amplatzer™ Cardiac Plug device with the lobe implanted at 10 mm from the ostium and the disc covering the entrance to the left atrial appendage (right).
Numerous registries attest to the efficacy and safety of the Watchman™ system and the ACP, but both require a not-inconsiderable implantation learning curve. The PROTECT AF study,12 the only randomized study to compare warfarin with LAA occlusion (Watchman™) in patients with NVAF, showed that LAA occlusion is no less successful than warfarin concerning the primary objective (combined cerebrovascular accident, systemic embolism, and cardiovascular or unexplained death) but showed a worrying rate of periprocedural events, with 4.4% incidence of severe pericardial effusion. This initial problem was attributed to the operator learning curve: with operator experience, the complication rate fell and the percentage of successful implantations improved significantly. Currently (for both devices) more than 95% of implantations are generally successful and the severe pericardial effusion rate is <2%.
TECHNICAL CONSIDERATIONSThe percutaneous LAA occlusion technique requires a team with experience in congenital/structural disease. Although some groups use only intracardiac echocardiography, or even angiographic control, to guide the procedure and avoid patient intubation, most centers use transesophageal echocardiography (TEE) under general anesthesia. Structurally, the LAA has very fine walls and a heterogeneous morphology that can hide microthromboses from the TEE. Operator experience is essential to minimize manipulation within the LAA and thus reduce the risk of perforation and periprocedural embolism. Two of the most important factors that minimize manipulation in the LAA are the following: a) a low posterior transseptal puncture that facilitates a frontal approach to the LAA, and b) a detailed study of the LAA, if possible with TEE and angiography, in order to establish the LAA morphology and obtain accurate measures that facilitate the selection of occluder size. It is recommended that measurements be taken at normal blood volume because LAA size can vary significantly as a function of the patient's level of hydration. During the procedure, patients tend to become dehydrated; therefore, it is recommended that left atrium pressure be determined and liquid administered to maintain >10 mmHg pressure before taking measurements.
This is a relatively new procedure and, even though TEE is the imaging standard, the ideal imaging mode remains unknown. The LAA morphology is oval in 80% of patients, so diameters usually vary because of the short and long axes. This difference can go unnoticed with 2-dimensional imaging techniques. As in procedures like percutaneous aortic valve replacement, 3-dimensional TEE or cardiac tomography can provide spatial information that may help optimize the implantation strategy and device size selection.
The significance of periprosthetic leaks during follow-up remains uncertain. A PROTECT AF study subanalysis found no relationship between leaks and the appearance of clinical events. In contrast, in studies of coronary cardiac surgery outcomes, incomplete LAA excision in patients who had surgical prophylactic LAA occlusion has been associated with a higher rate of cardioembolic events. As we wait for new studies to become available that may or may not confirm this relationship, we should try to prevent periprosthetic leaks. Hence, it is recommended that the device should be somewhat larger than TEE and angiographic measures would suggest, as this makes it possible to reduce the rate of residual leaks without increasing the risk of LAA wall rupture.13
Postprocedural antithrombotic recommendations are another controversial issue. In the PROTECT AF study, OAC were administered for 45 days postimplantation and suspended if the TEE showed adequate LAA occlusion.12 Recently, the ASAP study showed that 45 days of OAC treatment can replace dual antiplatelet therapy (100mg/day acetylsalicylic acid and 75 mg/day clopidogrel).14 Currently, most centers recommend dual antiplatelets for 1 to 3 months and indefinite antiplatelet monotherapy. Device thrombosis is an infrequent complication but has been described in both the Watchman™ and ACP devices. Although this complication is resolved by 2 weeks of anticoagulation therapy in most patients, the potential risk of embolism due to thrombus migration makes it one of the most feared complications. In the only Spanish series reported, an alarming rate of device thrombosis was observed in 14% of patients, in contrast with the <2% rate found in registries from around the world.15 This difference in the percentage of thrombosis could be due to a difference in the sensitivity of detection: in the Spanish registry, the TEE follow-up protocol was much longer (24 h and 1, 3, 6 and 12 months) than in most of the centers studied, where TEE was used for 3 to 6 months.15 Given that most cases of thrombosis occurred at =3 months and all were resolved by administering sodium heparin or enoxaparin for 2 weeks, determining the feasibility of 3 months of anticoagulation therapy or more exhaustive follow-up could be a future option, once the true incidence of device thrombosis has been clarified. In any case, multidisciplinary, individualized assessment is necessary in patients of this type, who sometimes present such a high bleeding risk that even short periods of anticoagulation therapy could be contraindicated.
CONCLUSIONS AND PROSPECTS FOR THE FUTUREEven though NVAF is already one of the most frequent diseases in our society, its prevalence is going to increase in the coming years due to the progressive aging of the population. For different reasons, between 30% and 50% of patients indicated for OAC are not taking them. Today, LAA occlusion constitutes an alternative for patients with NVAF and contraindications for anticoagulation therapy. LAA occlusion is an efficient and safe procedure, but it requires a team with experience in structural interventional cardiology and is associated with a substantial learning curve for the operator.
In the future, LAA occlusion could be an alternative to OAC treatment for all patients with NVAF. Although the PROTECT AF study has already proven that the efficacy of LAA occlusion is no less than that of anticoagulation therapy, the initial learning curve conditioned a high periprocedural complication rate. Two new randomized studies—PREVAIL and ACP—will specifically compare LAA occlusion with OAC treatment in patients with NVAF once the initial procedure learning curve has been overcome. The PREVAIL study compares the Watchman™ device with warfarin, and ACP compares the ACP device with warfarin and dabigatran. If the results of both studies show that LAA occlusion is no less successful than anticoagulation therapy in patients with NVAF, use of the procedure could be extended and it might become an alternative to anticoagulation therapy for patients with a lower risk profile.
CONFLICTS OF INTERESTXavier Freixa is proctor of St. Jude Medical.