Tricuspid valve disease is a frequent condition but is currently undertreated. A limited number of patients undergo an isolated surgical tricuspid repair, and this intervention is associated with poor outcomes, especially in patients with previous cardiac surgery. Most patients are only medically treated, despite the impact of severe tricuspid regurgitation on functional status and long-term survival. Transcatheter therapies represent a promising alternative for patients with severe tricuspid regurgitation and high surgical risk. In the last few years, several percutaneous alternatives have been developed for the treatment of functional tricuspid regurgitation. Imaging techniques play an indispensable role in patient selection, procedural guidance and follow-up. The current available transcatheter options for native tricuspid valve disease can be divided into 3 main groups: heterotopic caval valve implantation, annuloplasty devices, and coaptation devices. In patients with previous tricuspid valve surgery, transcatheter valve-in-valve and valve-in-ring procedures have been reported. This review provides a detailed analysis of the novel transcatheter alternatives for the treatment of tricuspid valve disease that have already been successfully implanted in humans, as well as the most important aspects of tricuspid valve anatomy and imaging assessment.
Keywords
Tricuspid valve (TV) disease has historically been neglected and its consequences often considered too late. One datum that supports this assertion is the fact that very few patients undergo cardiac surgery for isolated tricuspid disease.1 Most patients with significant tricuspid regurgitation (TR) are medically treated, and only 0.5% of them undergo TV repair or replacement.2 Some observational studies have shown that severe TR is independently associated with an increase in mortality,3,4 but in the absence of other significant valvular disease with surgical indication, it is often managed medically. Even in some aortic or mitral valve surgical interventions with concomitant severe TR, the latter is sometimes not repaired because of the classic misconception that TR always improves after left-sided heart valve surgery. In fact, 48% of patients show an increase in TR severity of at least 2 grades in the follow-up of mitral surgery.5 Following this evidence, the European Society of Cardiology recommends that moderate or severe TR should be repaired if there is another planned left-sided valve surgery.6 Moreover, even in the absence of significant TR, TV repair is indicated in patients undergoing left-sided valve surgery when the tricuspid annulus is already dilated. This recommendation aims to prevent the progression of the TR when the annulus is already dilated and the need for a subsequent surgical procedure.5 Isolated TV surgery is associated with a high in-hospital mortality rate, which can reach 10% in patients with previous left-sided valve surgery.7 In the particular case of redo tricuspid surgery after a first tricuspid repair, the in-hospital mortality rate can reach 35%.8 One of the main reasons for this high in-hospital mortality rate are late surgeries, when the right ventricle (RV) is irreversibly dilated and dysfunctional. Therefore, there is a clinical need for percutaneous therapies due to the large number of patients who are only medically managed due to a high-surgical risk.
The development of transcatheter techniques in structural heart disease, such as transcatheter aortic valve replacement or percutaneous edge-to-edge mitral repair, allows the treatment of high-surgical-risk patients and is one of the most important cardiology milestones in the present era. Nevertheless, among the predictors of mortality after a transcatheter edge-to-edge mitral repair, severe TR at baseline is the most important predictor.9,10 Furthermore, in patients undergoing transcatheter aortic valve replacement, moderate to severe TR is independently associated with increased 1-year mortality.11 These results underscore the need for tricuspid transcatheter devices, especially in the context of the growing transcatheter treatment of left-sided valve disease. Following the success of transcatheter interventions in aortic, pulmonic or mitral valve disease, recent years have witnessed the emergence of numerous percutaneous tricuspid techniques. This review presents the current status and directions of transcatheter therapies for TV disease, as well as the most important aspects of TV anatomy and TR assessment.
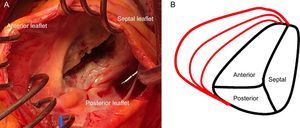
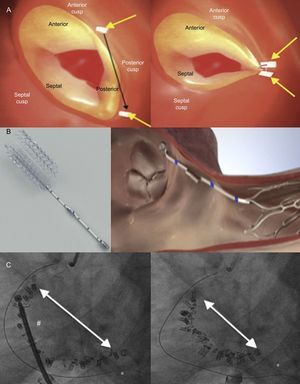
TRICUSPID VALVE ANATOMYThe normal TV is the right-sided atrioventricular valve in an anterior and inferior position in the heart, with the most apical situation among the 4 valves. The TV apparatus consists of a fibrous annulus surrounding 3 triangular leaflets, which are maintained by tendinous chords and respective RV papillary muscles. The 3 leaflets are designated according to their relative position in the heart: septal, anterior and posterior12 (Figure 1A). The TV is the largest of the 4 cardiac valves and has dissimilarities with its systemic counterpart in the left heart, the mitral valve, since the annular orifice area is about 20% larger than the mitral annulus area. The tricuspid annulus is complex with a saddle ovoid shape and a dynamic change during the cardiac cycle.13,14
Because the RV is a low-pressure chamber facing a low resistance pulmonary circulation, it demonstrates a heightened sensitivity to afterload change compared with the left ventricle.15 Because of its anatomy, the integrity of the TV apparatus is closely related to RV size and function, and RV volume and/or pressure overload can impair TV function.16 The tricuspid annulus is dynamic and it changes with varying loading conditions and during the cardiac cycle. The initial RV dilatation leads to tricuspid annulus dilatation, which is the dominant mechanism of functional TR. When functional dilatation occurs, not all leaflets play an equal role in TR: the annulus dilates in the septal to lateral direction, sparing the septal portion and spreading the coaptation point (Figure 1B). The annulus shape changes from elliptical to circular and results in a more planar shape with coaptation gaps that create TR jets.14 The anterior leaflet is the largest and is anchored to a single papillary muscle attached to the free wall of the RV, consequently being greatly affected by annular dilation.17 Hence, it is important to understand the complex relationships among the RV, the TV and the tricuspid annulus, which explain that functional TR is not a valvular disease but is rather the result of disease processes that alter the tricuspid annulus size as well as produce abnormalities in RV size and function, which thereby alter the mode of tricuspid leaflet coaptation and produce TV tethering.17
TRICUSPID REGURGITATION IMAGING ASSESSMENTThe most robust imaging technique used in clinical practice to explore the TV is echocardiography. The integration of the complex relationship between the RV and the TV apparatus requires the performance of multiple echocardiographic windows, most of the time from transthoracic echocardiography and sometimes from transesophageal echocardiography (TEE), with both 2-dimensional and 3-dimensional (3D) imaging.18,19
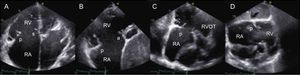
Transthoracic EchocardiographyThe recommended views for performing a comprehensive evaluation of the RV and TV apparatus have been outlined by the American Society of Echocardiography guidelines20,21 (Figure 2). Most of the parameters used for grading the severity of TR are qualitative or semiquantitative.22,23 A few reports have validated quantitative assessment of tricuspid regurgitant volume by the proximal isovelocity surface area method with a cutoff value ≥ 40mm2 for the effective regurgitant orifice area and ≥ 45mL for the regurgitant volume as sign of severe TR.24,25
Transthoracic imaging views for tricuspid valve evaluation. A: on-axis 4-chamber view. B: parasternal short axis view. C: on-axis 2-chamber view with the RV in the apex of the sector. D: subcostal view. a, anterior leaflet; p, posterior leaflet; s, septal leaflet; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract.
A new approach for assessing functional TR suggests taking into account the tricuspid annular size, the mode of leaflet coaptation and leaflet tethering, which are influenced by RV enlargement and dysfunction, instead of relying solely on TR severity.17 Current European and American guidelines suggest using the end-diastolic septolateral dimension from the transthoracic apical 4-chamber view to evaluate the size of the tricuspid annulus, with a diastolic dimension of ≥ 40mm (or > 21mm/m2) indicating severe tricuspid annulus dilation.6,26 The leaflet coaptation should also be analyzed carefully. Leaflet tethering is considered significant when the tethering distance is > 8mm or the tenting area is > 1.6cm2.27
The parameters most commonly used for assessment of TR are summarized in Table 1.28
Echocardiographic Assessment of Tricuspid Regurgitation Severity
| Parameters | Mild | Moderate | Severe |
|---|---|---|---|
| Qualitative | |||
| TV morphology | Normal or mildly abnormal leaflets | Usually abnormal leaflets | Severe valve lesions |
| Interventricular septal motion | Normal | Typically normal | Paradoxical/volume overload pattern |
| Color flow TR jet | Small RA penetration or not holosystolic | Moderate RA penetration or large penetration and late systolic | Deep RA penetration and holosystolic jet, or eccentric wall-impinging jet |
| Flow convergence zone | Not visible, transient or small | Intermediate in size and duration | Large throughout systole |
| CW TR jet density/contour | Faint/parabolic or partial contour | Dense, variable contour | Dense, triangular with early peaking contour |
| IVC size | Usually normal | Usually normal or mild dilation | Usually dilated with reduced respirophasic variability |
| RV and RA size | Usually normal | Usually normal or mild dilation | Usually dilated |
| Semiquantitative | |||
| Tricuspid annulus | < 40mm or 21mm/m2 | May be > 40mm or 21mm/m2 | > 40mm or 21mm/m2 |
| Color flow jet area, cm2 | Not defined | Not defined but < 10 | > 10 |
| Vena contracta, cm | Not defined | Not defined but < 0.7 | ≥ 0.7 |
| PISA radius, cm | ≤ 0.5 | 0.6–0.9 | > 0.9 |
| Hepatic vein flow | Systolic dominance | Systolic blunting | Systolic flow reversal |
| Tricuspid inflow | A-wave dominant and/or E-wave < 1 m/s | Variable | E-wave dominant and ≥ 1.0 m/s |
| Quantitative | |||
| EROA, mm2 | < 20 | 20-39 | ≥ 40 |
| Regurgitant volume, mL | < 30 | 30-45 | ≥ 45 |
CW, continuous wave; EROA, effective regurgitant orifice area; IVC, inferior vena cava; PISA, proximal isovelocity surface area; RA, right atrium; RV, right ventricle; TV, tricuspid valve; TR, tricuspid regurgitation.
Extract with permission from Rodés-Cabau et al.28
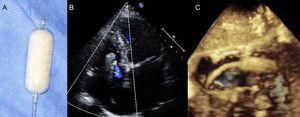
Transesophageal echocardiography allows imaging the TV with better spatial resolution and more windows for a comprehensive assessment in both 2-dimensional and 3D imaging.29 Having adjacent structures within the same volume during 3D imaging may help to identify leaflet anatomy (Figure 3). Standardized images from TEE are particularly important to assist interventional cardiologists during tricuspid transcatheter interventions.
Computed Tomography and Cardiac Magnetic Resonance ImagingAlthough echocardiography is the benchmark for the evaluation of TR, both computed tomography and cardiac magnetic resonance can provide additional information.
Because of its high spatial resolution, cardiac magnetic resonance can be additive to 3D echocardiography for both anatomic and functional analysis of the TV, annulus, and right-sided heart chambers.30
Computed tomography is useful for measuring the tricuspid annulus and can provide images of the RV morphology. Regarding the specific topic of transcatheter therapy, computed tomography provides information on the surrounding structures and their relationship with the device target zone. Furthermore, because right coronary artery (RCA) courses through the right atrioventricular groove, it is particularly important to assess its distance from the TV annulus in order to prevent a RCA injury during percutaneous annuloplasty procedures.31
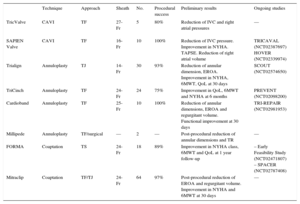
TRANSCATHETER THERAPIES FOR TRICUSPID REGURGITATIONOver the last few years, several transcatheter options have been specifically developed for the treatment of functional TR. Unlike aortic or mitral valve disease, there is, to date, no specific transcatheter heart valve (THV) available and only some preclinical experiences have been reported. Several challenges for the development of transcatheter tricuspid devices–such as the large dimension of the tricuspid annulus (> 40mm) and its nonplanar and elliptical shape–have been identified. Moreover, some characteristics of the RV represent additional challenges such as the slow flow, trabeculated structure with a thin ventricular wall, as well as the proximity to other structures like the RCA, the coronary sinus, the vena cava or the atrioventricular node.28 Some devices designed for mitral or aortic disease, such as the MitraClip (Abbott Vascular, Santa Clara, California, United States) or the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, California, United States), have been successfully adapted for the treatment of TR or its consequences, and other specific tricuspid devices have been developed. We can identify 3 different targets for the current tricuspid transcatheter therapies in the treatment of TR: implants of THV at the vena cava to reduce reverse backflow, percutaneous annuloplasty devices shortening annulus dimension, and devices improving leafltet coaptation and reducing the regurgitant orifice. Initial in-human experiences with these devices have been reported, and ongoing and future studies will evaluate the feasibility, safety, and efficacy of these new transcatheter options (Table 2). In patients with prior tricuspid repair or replacement and degenerated bioprostheses or annuloplasty ring failure, THV implantation using transcatheter aortic or pulmonic valves has been reported, becoming a promising novel alternative to redo surgery.
Percutaneous Treatments for Native Tricuspid Valve Disease
| Technique | Approach | Sheath | No. | Procedural success | Preliminary results | Ongoing studies | |
|---|---|---|---|---|---|---|---|
| TricValve | CAVI | TF | 27-Fr | 5 | 80% | Reduction of IVC and right atrial pressures | — |
| SAPIEN Valve | CAVI | TF | 16-Fr | 10 | 100% | Reduction of IVC pressure. Improvement in NYHA. TAPSE. Reduction of right atrial volume | TRICAVAL (NCT02387697) HOVER (NCT02339974) |
| Trialign | Annuloplasty | TJ | 14-Fr | 30 | 93% | Reduction of annular dimension, EROA. Improvement in NYHA, 6MWT, QoL at 30 days | SCOUT (NCT02574650) |
| TriCinch | Annuloplasty | TF | 24-Fr | 24 | 75% | Improvement in QoL, 6MWT and NYHA at 6 months | PREVENT (NCT02098200) |
| Cardioband | Annuloplasty | TF | 25-Fr | 10 | 100% | Reduction of annular dimensions, EROA and regurgitant volume. Functional improvement at 30 days | TRI-REPAIR (NCT02981953) |
| Millipede | Annuloplasty | TF/surgical | — | 2 | — | Post-procedural reduction of annular dimensions and TR | — |
| FORMA | Coaptation | TS | 24-Fr | 18 | 89% | Improvement in NYHA class, 6MWT and QoL at 1 year follow-up | – Early Feasibility Study (NCT02471807) – SPACER (NCT02787408) |
| Mitraclip | Coaptation | TF/TJ | 24-Fr | 64 | 97% | Post-procedural reduction of EROA and regurgitant volume. Improvement in NYHA and 6MWT at 30 days | — |
6MWT, 6-minute walk test; CAVI, caval valve implantation; EROA, effective regurgitant orifice area; IVC, inferior vena cava; NYHA, New York Heart Association; QoL, quality of life questionnaires; TAPSE, tricuspid annular plane systolic excursion; TF, transfemoral; TJ, transjugular; TR, tricuspid regurgitation; TS, trans-subclavian.
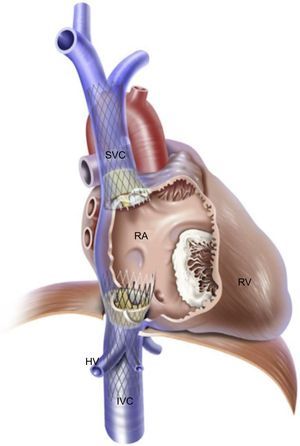
The rationale for this intervention is to reduce the regurgitant volume and pressure into the vena cava present in patients with severe TR that leads to hepatic, abdominal and peripheral congestion, thus reducing symptoms of right heart failure. Caval valve implantation has been performed via specifically designed devices such as the self-expandable TricValve (P+F Products + Features Vertriebs GmbH, Vienna, Austria) and the balloon-expandable SAPIEN transcatheter aortic valve. The TricValve consists of a pericardial tissue valve on a nitinol stent frame that can be implanted at the inferior vena cava alone or in combination with a specific TricValve for superior vena cava implantation (Figure 4).32 Lauten et al., 28,32,33 have reported acute hemodynamic and clinical improvements in patients implanted with TricValve at the cavoatrial inflow. A 27-Fr introducer is required for transvenous implantation.33 Preoperative inferior and superior vena cava sizing is mandatory and can be an exclusion criterion for this technique if there is a potential risk of valve embolization. The maximum size of this device is 43mm for the inferior vena cava and 38mm for the superior vena cava. Using the same concept, Laule et al.34 have reported the implantation of 29-mm-SAPIEN XT or SAPIEN 3 valves at the inferior vena cava. Before final valve implantation, a peripheral stent is deployed to create a landing zone. The main disadvantage of caval valve implantation is that this therapeutic concept does not reduce TR, only its consequences. No long-term data are available on the safety of the right atrium ventricularization and the persistent right atrium and RV overload due to persistent severe TR. Anticoagulation is recommended because the low flow in the vena cava could favor valve thrombosis. Three challenges must be taken into account in caval valve implantation procedures: the proximity of hepatic veins just below the diaphragm, the compliance and degree of dilation of the vena cavae, and the important anatomic variability of the superior vena cava, making double caval valve implantation a more technically demanding procedure.35 A high procedural success was reported with the 2 valvular systems, but 1-year outcomes showed a high mortality rate in these initial series of patients with prohibitive surgical risk and compassionate use.28 Insufficient data are available on long-term outcomes after device implantation. The safety and efficacy of SAPIEN valve implantation at the inferior vena cava is currently being studied in the TRICAVAL (NCT02387697) and HOVER (NCT02339974) trials.
Heterotopic caval valve implantation. Bicaval valve implantation with the self-expandable TricValve device at the inferior vena cava and superior vena cava. HV, hepatic veins, IVC, inferior vena cava; RA, right atrium; RV, right ventricle; SVC, superior vena cava. Reprinted with permission from Lauten et al.32
The pathophysiology of functional TR involves tricuspid annulus dilation. Progressive tricuspid annulus dilation occurs in its anteroposterior plane, which leads to a lack of leaflet coaptation. Tricuspid valve annuloplasty is the basis of current surgical therapy for functional TR13 and several percutaneous annuloplasty devices have been developed in recent years. These devices have the advantage of being based on a proven surgical background with good long-term outcomes. Moreover, these techniques preserve the anatomy of the TV, allowing future treatment options such as THV or percutaneous edge-to-edge repair if necessary.
TrialignThis device is based on the Kay surgical bicuspidization procedure (conversion of an incompetent TV into a competent bicuspid valve).36 The Trialign device (Mitralign Inc, Tewksbury, Massachusetts, United States) performs a transcatheter TV repair through the transjugular approach. The first-in-man tricuspid annuloplasty was reported in 2015 and used the mitral platform of this device (Mitralign)37 prior to the development of a specific tricuspid delivery system.
Two 14-Fr sheaths are implanted in the right jugular vein and a steerable wire delivery catheter is advanced across the TV and moved to the postero-anterior valve commissure under 3D TEE guidance. An insulated radiofrequency wire is advanced through the tricuspid annulus toward the right atrium and externalized in order to advance a pledget delivery catheter.28,37 Two polyester pledgets are anchored at the TV annulus in the posteroanterior and posteroseptal positions. In patients with a very large annulus, multiple pledgets can be implanted. Pledgets are then cinched together to obliterate the posterior leaflet and a dedicated lock system is delivered on the atrial side. As a consequence, the annular dimensions and the TR are reduced (Figure 5A).37
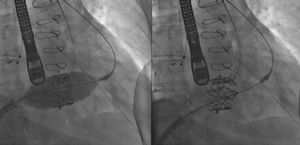
Percutaneous annuloplasty devices. A: Trialign: suture pledgets (yellow arrows) anchored at the TV annulus (left). Reduction of the tricuspid annular dimension after plication of the posterior leaflet (right). Reprinted with permission from Schofer et al.37 B: TriCinch device (left). Illustration of TriCinch implantation (right). Reprinted with permission from Rodés-Cabau et al.28 C: Cardioband: Fluoroscopic images after device implantation, before (left) and after (right) cinching, with significant reduction of tricuspid annulus diameter. *, Coronary wire in the right coronary artery. #, Cardioband cinching catheter. Reprinted with permission from Schueler et al.45
To date, more than 30 patients worldwide have been treated with this device. A post-procedural mean annular reduction of 37% and a regurgitant orifice area reduction of 59% have been reported. The US early feasibility study SCOUT I38 included 15 patients with procedural success in all patients and 30-day technical success in 12 patients (3 cases of single pledget dehiscence). A significant reduction of the tricuspid annulus diameter and the regurgitant orifice area as well as an improvement in the patients’ symptoms (functional status, 6-minute walk test, and quality of life measures) were observed at the 30-day follow-up.38 These preliminary results will be evaluated in a larger cohort of patients in the European Union CE mark study.
TriCinchThe TriCinch (4Tech Cardio, Galway, Ireland) is a transcatheter device designed to reduce functional TR by reducing the annular dimension and restoring leaflet coaptation.39 It consists of a corkscrew, a Dacron band, and a self-expandable nitinol stent with 4 available sizes, from 27 to 43mm40 (Figure 5B).28 The procedure is usually performed under general anesthesia and with fluoroscopic, TEE and intracardiac echocardiography guidance. However, a successful procedure under conscious sedation and with only fluoroscopic and intracardiac echocardiography guidance was recently reported.41 A guidewire is placed in the RCA due to the risk of coronary artery injury during TriCinch implantation. A 24-Fr introducer sheath is placed in the right femoral vein and an 18-Fr steerable catheter is delivered to the tricuspid annulus. A stainless steel corkscrew is anchored in the TV annulus next to the anteroposterior commissure. The stent delivery system is locked to the corkscrew via the Dacron band, and tension is applied to reduce the septolateral diameter and TR severity by pulling the system toward the inferior vena cava. Finally, the stent is deployed in the inferior vena cava to maintain the tension applied.28,39,40 The proximity of the RCA to the TV annulus and the lack of annular shelf are potential limitations to this technique, hence requiring careful preprocedural analysis. Preoperative computed tomography is mandatory to evaluate the tissue quality and the risk of detachment or dehiscence and RCA injury.42
The ongoing PREVENT study (NCT02098200), a multicenter CE mark trial, is evaluating the safety and performance of the TriCinch system for functional TR repair. To date, 24 patients have been enrolled in the study and 18 successful implants have been performed (75%). Two hemopericardium and 4 postoperative annulus anchor detachments were reported. A TR reduction has been achieved in 94% of the successfully implanted patients. At the 6-month follow-up, improvements in quality of life were reported and 75% of patients showed functional class I or II.43 This initial experience confirms that tricuspid annular remodeling with the TriCinch system is feasible and safe in selected patients. Moreover, TriCinch preserves the native anatomy and allows for future treatment options. The second generation of the TriCinch device has a new anchoring system with a hemispiral shape which is deployed in the pericardial space. The main advantage of this anchoring system is the independence from the RCA location and tissue quality.42
CardiobandThe Cardioband system for the treatment of the TV (Valtech Cardio, Or-Yehuda, Israel)44 is a percutaneous annuloplasty ring based on the CE-approved Cardioband device for mitral regurgitation. This Dacron adjustable band is fixed in a supra-annular position, similar to a surgical annuloplasty, and allows for bidirectional adjustability (avoiding over-cinching and post-procedural transvalvular gradients) up to a size of a 28-mm surgical ring (Figure 5C).45 The Cardioband delivery system for TR requires a 25-Fr transfemoral introducer sheath. For orientation and safety reasons, a guidewire is placed in the RCA.45 The procedure is performed under fluoroscopy and 3D TEE guidance. The Cardioband is fastened to the annulus by 17 stainless steel anchors with a length of 6mm that are implanted from the anterior to the posterior tricuspid annulus. Once the anchors are fixed, the device is cinched and the tricuspid annular dimensions are significantly reduced. Important advantages of this technique include its reversibility and its ability to be adapted to the tricuspid annular geometry,46 distributing the annular reduction across the annulus, thus reducing the stress on the anchoring sites. The preliminary results of the TRI-REPAIR-study (NCT02981953) were recently reported in 10 patients, with a significant reduction in annulus diameter and TR regurgitation volume along with improvements in functional status at 30 days.47 The TRI-REPAIR study will assess the safety and technical success of the Cardioband system for the treatment of symptomatic functional TR in 30 patients. Further studies should elucidate the durability and long-term clinical outcomes of this transcatheter annuloplasty device.
MillipedeThe Millipede (Millipede Inc, Santa Rosa, California, United States) annuloplasty device consists of a semirigid, adjustable, complete ring that can be implanted by the surgical or transfemoral approach. It has the advantage of being repositionable and retrievable before deployment and provides a stable annular reduction.48 It presents an interruption for atrioventricular node in order to reduce the risk of atrioventricular block.39 This device has been implanted in the mitral position in 9 patients, 2 of whom underwent a Millipede implantation in the mitral and TV. The reduction of the tricuspid annulus diameter in these 2 patients reached 42% and 45%, respectively, and post-procedural TR grade was 0.49
Coaptation DevicesFORMAThe FORMA device (Edwards Lifesciences) is designed to reduce functional TR by occupying the regurgitant orifice and providing a platform for native leaflet coaptation.50 It consists of a spacer and a rail. The rail tracks the spacer into position and is distally anchored at the RV apex, perpendicular to the tricuspid annulus plane. The spacer is a foam-filled balloon which is positioned in the regurgitant orifice under fluoroscopic and 3D TEE guidance (Figure 6). Two spacer sizes are available: 12 and 15mm, requiring an introducer sheath of 20 and 24-Fr, respectively, at the left axillary or subclavian vein. The final spacer size is achieved by passive expansion via 8 holes in the spacer shaft.28 Once the spacer is placed in the optimal position to reduce TR, the device is proximally locked and the excess rail length is placed inside a subcutaneous pocket, using a similar technique to a standard pacemaker implantation. This device has been implanted in 18 patients on a compassionate use basis, with 2 unsuccessful implants due to 1 acute anchor dislodgment and 1 RV perforation. At 1-year follow-up, 1 rehospitalization for heart failure and no deaths were reported. Patients implanted with the FORMA device have shown an improvement in New York Heart Association functional class, walking distance, and quality of life questionnaires.51 A certain degree of TR reduction has been reported in all patients, but post-procedural echocardiographic evaluation of residual TR severity is particularly difficult due to the presence of multiple small jets. No iatrogenic tricuspid stenosis has been reported, with a mean transtricuspid gradient of 1.2mmHg.50 Patients with very large coaptation defects may constitute a potential limitation for the use of this device, as the current 15-mm spacer could be insufficient to achieve a significant TR reduction. Patients with pacemaker leads can be implanted with the FORMA device, but this population usually has an asymmetric regurgitant orifice, hence TR reduction could be more challenging. Two trials–the US Early Feasibility Study (NCT02471807) and the EU/Canada SPACER trial (NCT02787408)–will evaluate the safety and efficacy of the FORMA device in 30 and 75 patients, respectively.
MitraClip in the Tricuspid ValveTranscatheter TV edge-to-edge repair using the MitraClip (Abbott Vascular) system is a feasible alternative for patients with severe TR.52,53 The MitraClip in the tricuspid position mimics the surgical edge-to-edge “clover” technique, which has been validated for the treatment of complex TR, showing satisfactory results at long-term follow-up.54 The MitraClip device consists of a 4-mm wide cobalt-chromium, polyester-covered implant with 2 arms that can be opened and closed to grasp the valve lealflets.16 Tricuspid edge-to-edge repair can be performed by the transjugular or transfemoral approach. In a recent multicenter study, 64 consecutive patients underwent MitraClip implantation with a 97% procedural success. Two or more clips were needed in 42% of the patients. Significant reductions in effective regurgitant orifice area and regurgitant volume were observed. At 30-days’ follow-up, this observational study reported a significant improvement in the New York Heart Association functional class and 6-minute walk test.55 Despite the broad experience with this device in the mitral valve, edge-to-edge tricuspid repair remains a challenging procedure. The main specific challenges are secondary to the increased anatomic variability and the large coaptation gaps, with increased difficulty for leaflet grasping, often requiring multiple clips. Additional challenges are the difficulty in steering the MitraClip delivery system into the right atrium perpendicular to the TV plane, and the risk of clip entrapment in the subvalvular apparatus. The procedure is guided by TEE but, unlike the mitral valve, the visualization of the TV leaflets can be suboptimal in some patients.53 A new version of the device with longer arms (MitraClip XT) as well as a specific tricuspid delivery system are needed to improve the feasibility and outcomes of this technique over the next few years.56
TRANSCATHETER TRICUSPID THERAPIES AFTER TRICUSPID VALVE REPAIR OR REPLACEMENT FAILURETricuspid Valve-in-valve and Valve-in-ringPatients with previous TV repair or replacement who require a tricuspid reintervention have a prohibitive surgical risk, reaching an in-hospital mortality of 35%.8 Up to 17% of patients undergoing tricuspid annuloplasty have severe TR at 5-years’ follow-up.57 The use of THV implantation for the treatment of degenerated tricuspid bioprostheses or failing annuloplasty rings has been reported. Transcatheter heart valve has been successfully implanted by the transatrial, transjugular or transfemoral approaches.48,58 Patients with prosthethic dysfunction and high surgical risk or patients with congenital heart disease and a history of several tricuspid interventions are good candidates for THV implantation.59 Two different THV have been successfully implanted during tricuspid valve-in-valve or valve-in-ring procedures: the SAPIEN transcatheter aortic valve and the Melody valve (Medtronic, Minneapolis, Minnesota, United States). In the case of the valve-in-ring interventions, semirigid or flexible rings seem to be more favorable than rigid and open rings, as it is easier to circularize their oval shape during THV deployment, thus reducing the risk of paravalvular leak48 (Figure 7).60 Experience with the tricuspid valve-in-ring is limited, and the largest multicenter registry reported only 20 valve-in-ring implantations with a high rate of significant paravalvular leaks.61
Tricuspid valve-in-ring. Transcatheter tricuspid valve-in-ring implantation using the SAPIEN XT valve in a patient with severe tricuspid regurgitation and prior tricuspid valve repair with a 32-mm Carpentier-Edwards (Edwards Lifesciences, Irvine, California, United States) annuloplasty ring. Reproduced with permission from Cabasa et al.60
A large multinational registry of tricuspid valve-in-valve procedures included 152 patients, with a high procedural success rate of 98.7%.62 Rapid ventricular pacing is required for valve deployment, but a temporary pacemaker lead cannot be placed in the RV to avoid a jailed catheter. Two alternatives for ventricular pacing have been reported: a temporary lead in the coronary sinus or direct pacing using the wire as a lead at the apex of the RV.48 In patients with a previous pacemaker, THV can be implanted without removing the pacemaker lead. Tricuspid valve-in-valve should be considered for patients with degenerated TV bioprostheses and high surgical risk. Further studies will determine the long-term durability of SAPIEN and Melody valves in the tricuspid position.48,62
FUTURE DEVICES AND DIRECTIONSSeveral new devices are currently under preclinical development, such as the TRAIPTA (Transatrial Intra-pericardial Tricuspid Annuloplasty) device which has already been implanted in an animal model.63 After entering the pericardial space, the device encircles the ventricles and is tightened, thus achieving a reduction of the tricuspid annulus dimension secondary to the external compression. The intrapericardial access site is closed with an atrial septal occluder at the end of the procedure.
The first experience with a transcatheter TV implantation was reported by Boudjemine et al., 64 more than 10 years ago in 8 ewes with a normal TV. Nevertheless, due to the large dimension of the tricuspid annulus, its oval shape and the lack of a rigid landing zone, as well as other anatomical considerations, no transcatheter tricuspid replacement has yet been performed in humans. Two promising TV systems are currently under development: the TriCares (TriCares GmbH, Aschheim, Germany) and NaviGate (NaviGate Cardiac Structures Inc, Lake Forest, California, United States).
Imaging techniques play a key role in patient selection and procedure planning, identifying potential challenges for each patient. The development of transcatheter techniques should be accompanied by new advances in imaging techniques adapted to the specific requirements of the different transcatheter devices. Experienced 3D TEE operators are required for the guidance of the transcatheter tricuspid procedures, having a direct impact on their success. The use of intracardiac echocardiography will probably increase in the next few years, allowing percutaneous tricuspid repair or replacement without general anesthesia.
Present and future transcatheter devices should demonstrate their safety and feasibility, as well as consistent results and durable outcomes. Randomized studies are needed to demonstrate their superiority over standard medical therapy in high surgical risk patients. In the coming years, some new percutaneous devices and transcatheter valves will probably undergo their first-in-man procedures, adding more alternatives for TR treatment. As already performed in mitral valve disease, some of tricuspid transcatheter devices could also be combined, such as annuloplasty and coaptation devices. Future studies should elucidate which is the most suitable transcatheter option for specific patient characteristics such as severe RV dysfunction or severe annulus dilation.
CONCLUSIONSSevere functional TR is still an undertreated valve disease, despite its impact on long-term survival. Surgical annuloplasty remains the gold standard for TV disease, but a very limited number of patients undergo isolated tricuspid repair. Most patients are not referred for tricuspid surgery and are only medically managed due to their prohibitive surgical risk. Moreover, nowadays an increasing number of patients undergo transcatheter interventions for mitral or aortic disease, but the presence of a concomitant untreated severe TR worsens the long-term results of these interventions. Therefore, there is a need for novel devices allowing transcatheter treatment of TV disease. In recent years, several percutaneous tricuspid devices have emerged and are currently being evaluated in clinical studies. Furthermore, additional specific devices and probably transcatheter TV will also be available in the near future. In view of the different treatment options currently under development and the renewed interest in the TV disease, we can affirm that the TV will no longer be the “forgotten” valve.
CONFLICTS OF INTERESTNone declared.
.