The Micra transcatheter pacing system has shown high effectiveness and a lower complication rate than conventional transvenous pacemakers. However, the benefit of the device is unknown in the very old population (≥ 90 years). The aim of this study was to evaluate the safety and effectiveness of Micra in patients ≥ 90 years.
MethodsWe present a prospective observational study with consecutive patients aged >70 years who underwent implantation of a Micra pacemaker system. Patients were divided into 2 groups: ≥ 90 and<90 years.
ResultsThe Micra system was implanted in 129 patients, of whom 41 were aged ≥ 90 years and 88<90 years. The device was successfully implanted in 40 (97.6%) patients ≥ 90 years and in 87 (98.9%) patients<90 years (P=.58). An adequate position was achieved with need for ≤ 2 repositions in 97.5% and 91.9% of patients, respectively (P=.32). Procedure time (26.1 ±11.6 vs 30.3 ±14.2minutes; P=.11) and fluoroscopy time (6.4 ±4.7 vs 7.2 ±4.9minutes; P=0.41) were similar in the 2 groups. There were 3 major complications (2.3%), all in the group aged<90 years: 1 cardiac perforation, 1 femoral hematoma, and 1 femoral pseudoaneurysm. Thirteen patients aged ≥ 90 years (31.7%) and 16 patients aged <90 years (18.2%) died during a mean follow-up of 230±233 days and 394±285 days, respectively. There were no device-related deaths. No infection, dislocation or migration of Micra were observed. The electrical performance was optimal at follow-up.
ConclusionsThe Micra leadless pacing system seems to be safe and effective in patients older than 90 years. It may be considered a reasonable alternative to conventional transvenous pacing in this population.
Keywords
Longer life expectancy and population aging have increased the number of implantations performed in very old patients (≥ 90 years), representing more than 9% of the total pacemaker implantations in some registries.1 A higher risk of complications with conventional transvenous pacemakers has been reported for older patients. The risk may be even higher if reintervention is needed.2–6
Leadless pacing has emerged as a safe and effective alternative to conventional pacemakers.7–9 In addition, this new technology avoids the complications classically related to the leads and the subcutaneous pocket. The Micra leadless transcatheter pacing system (TPS, Model MC1VR01, Medtronic plc, Mounds View, Minnesota, United States) has been evaluated in a large pivotal trial and in the Micra TPS Post-Approval Registry. The mean age of the study population in these trials was 75.9±10.9 and 75.1±14.2 years, respectively.7,8 Since very old patients were poorly represented, it is not known whether the benefit observed with the Micra system also extends to the oldest population.
The objective of this study was to evaluate the efficacy and safety of the Micra TPS in the very advanced age population (≥ 90 years) from our series compared with younger patients (< 90 years) at the time of implantation and during the follow-up.
METHODSWe started the leadless pacemaker Micra implantation program at our institution in June 2015. We present a prospective nonrandomized single-center study to evaluate the safety and efficacy of the Micra leadless pacemaker system in consecutive patients older than 70 years with a guideline-based indication for single-chamber ventricular pacing.
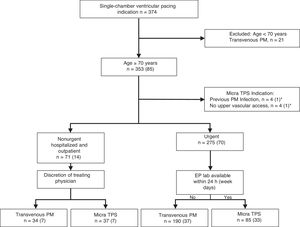
The decision to implant a Micra TPS or a single-chamber conventional transvenous pacemaker was based on clinical and care efficiency criteria. A Micra TPS was preferred for patients with previous pacemaker infection and/or absence of upper vascular access. Patients with urgent need of pacing routinely underwent a Micra TPS implantation if the electrophysiology laboratory was available within 24 hours after the indication, otherwise the patient received a conventional device in the cardiac surgery operating room. In non-urgent hospitalized patients and outpatients, the decision to implant a Micra TPS or a transvenous pacemaker was left at the discretion of the treating physician. There was no upper age limit for the implantation of the device and patients older than 90 years were routinely implanted from the beginning of the program. No patient was rejected for Micra implantation due to advanced age or comorbidity issues. A flowchart with the decision-making process between Micra and conventional single-chamber transvenous pacemaker is shown in Figure 1.
Flowchart of the decision-making process between the Micra and conventional transvenous pacemaker in patients with an indication for single-chamber ventricular pacing. The values in brackets represent the number of patients aged ≥ 90 years. EP lab, electrophysiology laboratory; PM, pacemaker; TPS, transcatheter pacing system. Figures in parentheses indicate patients aged ≥ 90 years. *One patient aged ≥ 90 years had both a previous pacemaker infection and absence of upper vascular access.
Patients were categorized according to age into 2 groups: ≥ 90 years and <90 years. The study was approved by the Ethics Institutional Committee on Human Research and all patients provided written informed consent prior to implantation. The primary efficacy endpoint at the implantation procedure was the achievement of a pacing capture threshold of ≤ 1.0 V at a pulse width of 0.24 ms. The efficacy endpoint during follow-up was defined as a pacing threshold of <1.5 V at 0.24 ms. The following major complications were defined, according to the Micra Investigational Device Exemption criteria: events resulting in death, permanent loss of device function as a result of mechanical or electrical dysfunction, hospitalization, prolongation of hospitalization by at least 48 hours, or system revision.8
Implantation procedureOral anticoagulation was stopped prior to implantation: vitamin K antagonists were generally withheld until the INR was ≤ 2 and nonvitamin K oral antagonists were stopped 24 hours before the procedure.
All interventions were performed with patients under conscious sedation. The Micra implantation was performed according to the standard technique described elsewhere.10 Venous femoral access was obtained by ultrasound-guided puncture and a 27-Fr introducer was advanced into the right atrium. The delivery system and the device were then advanced through the introducer and positioned in the septum of the right ventricle. Either an apical-septal or midseptal positions were targeted for implantation. Dye injection was routinely performed to confirm the correct apposition of the delivery catheter to the septum. The device was then deployed and affixed to the myocardium through the 4 nitinol tines. After verification of adequate electrical measurements, the “pull-and-hold maneuver” was routinely performed to confirm the correct anchoring of the device to the myocardium. When electrical measurements or device fixation were not appropriate, the device was repositioned.
An echocardiogram, chest X-ray and electrocardiogram as well as assessment of electrical parameters were performed 24 hours after implantation.
Follow-upFollow-up was performed at 7 days, and 1, 3, 6 and 12 months after implantation. Subsequent annual follow-ups were then performed. Patients with high pacing threshold at implantation (> 1.0 V at 0.24 ms) or during follow-up (≥ 1.5 V at 0.24 ms) were routinely included in the remote follow-up program, with monthly visits until pacing threshold stabilization, and then every 4 months.
Statistical analysisContinuous data are expressed as mean±standard deviation or median (interquartile range) when appropriate. Categorical or ordinal variables are expressed as percentages. All continuous data were tested using the 1-sample Kolmogorov-Smirnov test against a normal distribution. Comparison between groups was made using the Student t-test, the Mann-Whitney U test and the chi-square test, according to the type of variable and its distribution. A P value <.05 was considered statistically significant. The analysis was conducted with the IBM SPSS Statistics version 21.0 (SPSS, Chicago, Illinois).
RESULTSBaseline characteristicsA Micra pacemaker was implanted in 129 of 353 patients aged ≥ 70 years with an indication for a single-chamber pacing device in our institution during the study period: 41 (31.8%) in patients aged 90 (92.9±2.4) years or older and 88 (68.2%) in patients younger than 90 (83.9±4.1) years. Implantations for previous transvenous pacemaker infection and extraction were performed in 1 (2.4%) patient ≥ 90 years and in 3 (3.4%) patients <90 years. Implantations due to lack of vascular access were performed in 1 (2.4%) patient ≥ 90 years and in 3 (3.4%) patients <90 years. In patients with no specific indication for a leadless device (no previous device infection and/or absence of upper vascular access), Micra implantation was urgent and performed within 24 hours from indication in 85 patients (33 aged ≥ 90 years), and was non-urgent in 37 patients (7 aged ≥ 90 years) (Figure 1).
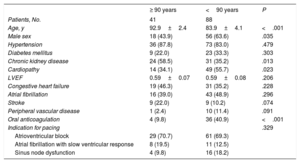
The baseline characteristics of the study population are shown in Table 1. The older group showed a higher proportion of women (56% vs 36%), a higher prevalence of chronic kidney disease (59% vs 35%), a lower prevalence of structural heart disease (34% vs 56%), and fewer indications for chronic anticoagulant therapy (10% vs 41%).
Baseline characteristics of the study population
| ≥ 90 years | <90 years | P | |
|---|---|---|---|
| Patients, No. | 41 | 88 | |
| Age, y | 92.9±2.4 | 83.9±4.1 | <.001 |
| Male sex | 18 (43.9) | 56 (63.6) | .035 |
| Hypertension | 36 (87.8) | 73 (83.0) | .479 |
| Diabetes mellitus | 9 (22.0) | 23 (33.3) | .303 |
| Chronic kidney disease | 24 (58.5) | 31 (35.2) | .013 |
| Cardiopathy | 14 (34.1) | 49 (55.7) | .023 |
| LVEF | 0.59±0.07 | 0.59±0.08 | .206 |
| Congestive heart failure | 19 (46.3) | 31 (35.2) | .228 |
| Atrial fibrillation | 16 (39.0) | 43 (48.9) | .296 |
| Stroke | 9 (22.0) | 9 (10.2) | .074 |
| Peripheral vascular disease | 1 (2.4) | 10 (11.4) | .091 |
| Oral anticoagulation | 4 (9.8) | 36 (40.9) | <.001 |
| Indication for pacing | .329 | ||
| Atrioventricular block | 29 (70.7) | 61 (69.3) | |
| Atrial fibrillation with slow ventricular response | 8 (19.5) | 11 (12.5) | |
| Sinus node dysfunction | 4 (9.8) | 16 (18.2) |
LVEF, left ventricular ejection fraction.
The data are expressed as mean±standard deviation or No. (%)
Advanced atrioventricular block was the main indication for cardiac pacing in both groups (70.7% in patients aged ≥90 years and 69.3% in those aged <90 years), followed by atrial fibrillation with slow ventricular response (19.5% vs 12.5%) and sinus node dysfunction (9.8% vs 18.2%). In 2 (4.9%) patients aged ≥ 90 years and in 9 (10.2%) patients aged <90 years, the indication was an atrioventricular conduction disorder following TAVI implantation.
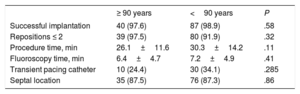
Implantation procedure outcomesThe main implantation procedure characteristics are shown in Table 2. The Micra pacemaker was successfully implanted in 40 of the 41 (97.6%) patients aged ≥ 90 years and in 87 of the 88 (98.9%) patients aged <90 years (P=.58). The 2 device implantation failures were due to important iliocaval venous tortuosity, which prevented the advance of the 27-Fr introducer in a patient aged ≥ 90 years, and to cardiac perforation in a patient aged <90 years. Procedure and fluoroscopy times were similar in the 2 groups. The implantation was successful after the first deployment of the device in 35 (87.5%) patients aged ≥ 90 years and in 58 (66.7%) aged <90 years (P=.01). No differences were found in the number of repositions required during the implantation: ≤ 2 repositions in 97.5% of the patients aged ≥ 90 years and in 91.9% of those aged <90 years (P=.32).
Implantation procedure characteristics
| ≥ 90 years | <90 years | P | |
|---|---|---|---|
| Successful implantation | 40 (97.6) | 87 (98.9) | .58 |
| Repositions ≤ 2 | 39 (97.5) | 80 (91.9) | .32 |
| Procedure time, min | 26.1±11.6 | 30.3±14.2 | .11 |
| Fluoroscopy time, min | 6.4±4.7 | 7.2±4.9 | .41 |
| Transient pacing catheter | 10 (24.4) | 30 (34.1) | .285 |
| Septal location | 35 (87.5) | 76 (87.3) | .86 |
The data are presented as mean±standard deviation or No. (%).
Electrical measurements at implantation are shown in Table 3. Thirty-nine (97.5%) patients aged ≥ 90 years and 83 (95.4%) of those aged <90 years had a threshold ≤ 1.0 V at 0.24 ms at implantation. Only 1 patient in the group aged ≥ 90 years and 2 in the group aged <90 years had a pacing threshold ≥ 1.5 V at 0.24 ms at implantation (1.5 V; 4.0 V and 1.5 V at 0.24 ms, respectively). In the patient with 4 V, the threshold elevation was observed early after implantation, after removal of the femoral introducer.
Procedure related complicationsComplications at implantation and within 30 days after implantation are shown in Table 4. Cardiac perforation requiring emergent cardiovascular surgery occurred in a patient aged <90 years (0.8%), an 83-year-old woman, early at the beginning of the study (patient #7). There were 2 relevant vascular complications (1.5%): 1 pseudoaneurysm and 1 femoral hematoma, both in the group aged <90 years. The pseudoaneurysm originated in the femoral access used for a temporary pacing catheter, contralateral to the access used for the Micra implantation and therefore not directly related to it. No major complications occurred in the group ≥ 90 years. Length of hospital stay, from pacemaker implantation indication to hospital discharge was similar in both groups (3.0 days [IQR 2.0-5.5] for patients ≥ 90 years, and 3.0 day [IQR 1.0-9.0] for patients <90 years; P=.95). There were no deaths related to the device implantation.
Major complications at implantation and within 30-day after implantation
| ≥ 90 years | <90 years | Total | |
|---|---|---|---|
| Patients, No. | 41 | 88 | 129 |
| Total major complications | 0 | 3 (3.4) | 3 (2.3) |
| Events at groin puncture site | 0 | 2 (2.3) | 2 (1.5) |
| Incision site hematoma | 0 | 1 (1.1) | 1 (0.8) |
| Pseudoaneurysm | 0 | 1 (1.1) | 1 (0.8) |
| Cardiac perforation | 0 | 1 (1.1) | 1 (0.8) |
The data are expressed as No. (%).
The mean length of follow-up was 342±279 days for the entire population, 230±233 days for patients aged ≥ 90 years and 394±285 days for those aged <90 years. There were 29 deaths during follow-up: 13 in the group aged ≥ 90 years (31.7%) and 16 in the group aged <90 years (18.2%), none of them related to the device. Most deaths (93.1%) were due to noncardiovascular causes. One patient in each group died from heart failure, one due to severe aortic valve stenosis (≥ 90 years), and another due to severe mitral regurgitation (< 90 years); none of these patients were considered suitable for valve replacement.
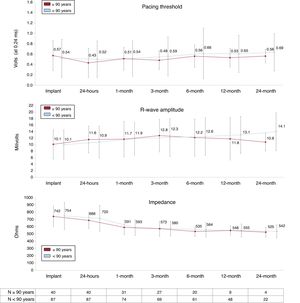
No differences were observed in electrical parameters between the 2 study groups during follow-up (Figure 2). All patients aged ≥ 90 years and 92.6% of those aged <90 years remained stable with a pacing threshold <1.5 V at 0.24 ms for more than 3 months after implantation.
The 2 patients aged <90 years with high pacing threshold at implantation showed a reduction in this parameter during follow-up: the patient with a threshold of 1.5 V showed a reduction in this parameter at the first month and it remained below 1.0 V after 2 years; in the other patient, with a pacing threshold of 4 V early after implantation, a reduction to 2.88 V was documented 24 hours after implantation and a progressive increase was then observed to 4.38 V after 6 months of follow-up. The only patient in the group aged ≥ 90 years with a high pacing threshold at implantation (1.5 V) died within the first month due to intestinal ischemia.
In contrast, 4 patients aged <90 years with an adequate pacing threshold at implantation (< 1.0 V), exhibited a progressive increase in this parameter during follow-up: in 1 patient the increase was transient, reaching 1.75 V at 3 months and subsequently decreasing below 1.5 V; in the other 3 patients, the pacing threshold progressively increased during the first year up to 2.88 V, 1.75 V and 1.63 V at 0.24 ms, respectively. Importantly, all these patients underwent remote follow-up surveillance and no reintervention was needed in any of them.
There were no device dislocations needing reintervention, migration nor infection during follow-up.
DISCUSSIONWe present the first study that evaluates the performance of the Micra leadless pacemaker in very advanced age patients. We have demonstrated that the safety and effectiveness of the Micra pacemaker in patients older than 90 years were comparable to that in younger patients in our study as well as in other series reported in the literature.7,8,10,11
Our data show a high rate of implantation success in both groups (97.6% in patients ≥ 90 years and 98.9% in <90 years), similar to the success rate reported in the pivotal trial and in the Micra TPS postapproval registry (99.2% and 99.6%, respectively).7,8 Therefore, very old age does not seem to limit the implantation procedure. This is supported by the comparable procedure duration, fluoroscopy time and need for device repositioning observed in our patients. Remarkably, the interventions were performed only under conscious sedation and local anesthesia in all patients, highlighting the good tolerance to Micra implantation procedure in the very old population.
In this study, the low incidence of significant complications related to Micra implantation extended to patients of very advanced age, who showed no significant adverse events. The cardiac perforation reported in our study occurred in the seventh patient of the series, from the group aged <90 years, and therefore within the earliest phase of the team's learning curve. The absence of any cardiac damage in the group of patients aged ≥ 90 years is important, considering that age has been previously associated with an increased risk of perforation, for both transvenous and Micra leadless pacemakers.6,7,12 A septal rather than apical position was generally preferred for Micra implantation throughout the study, and the septal implantation rate in the group of patients aged ≥ 90 years was much higher than that reported in the pivotal study (87% vs 33%).11 Our emphasis on implanting the device in a septal position in the right ventricle, avoiding the true apex, probably contributed to such a low rate of cardiac perforation specifically in the group aged ≥ 90 years. Although the rate of cardiac damage reported in the pivotal study was higher than that previously observed with conventional pacemakers (1.5% vs 0.47-1%), this difference could possibly be reduced by routine Micra implantation in septal positions.3,11,12 Only 2 relevant vascular complications occurred (1.5%), 1 pseudoaneurysm and 1 femoral hematoma, none in the group aged ≥ 90 years. Of note, the pseudoaneurysm originated in the femoral access used for a temporary pacing catheter, contralateral to the access used for the Micra implantation and therefore not directly related to it. Routine use of ultrasound for vascular access probably contributed to the lack of major vascular complications in the eldest group, as it helps ensure precise puncture in the common femoral vein, avoiding inadvertently crossing small arterial branches. Moreover, the use of a conservative anticoagulation protocol seems safe in this setting and probably helps to avoid serious groin complications.
Micra also exhibited a high safety profile in very old patients during follow-up, with no device-related infections, no device dislodgement or embolization, no need for reintervention in any patient and no device-related deaths.
The Micra showed an optimal electrical performance in the very advanced age population, comparable to that observed in the younger group. Pacing threshold at implantation was low in most patients, ≤ 1 V at 0.24 ms in 97.5%, and remained stable during follow-up, with no need for reintervention in any of the patients. Interestingly, patients with high pacing threshold at implantation or during early follow-up may improve or even normalize this parameter during the follow-up.
Some clinical characteristics differed between the groups, with patients ≥ 90 years having a higher prevalence of female sex and chronic kidney disease, and a lower prevalence of structural heart disease. These differences were probably due to the lower overall probability of survival, and thus of reaching the nonagenarian age, in patients who are male or who have significant structural heart disease.
Finally, noncardiovascular mortality in the study population was high during follow-up, similar to that reported by other studies in nonagenarians who undergo conventional transvenous pacemaker implantation.13 This observation and the current cost of the device raise the question of the cost-effectiveness of Micra TPS in such an elderly population, despite the potential clinical benefit. We believe that the cost reduction that usually occurs after a certain time with new medical technology will probably make this issue less relevant in the future, with greater prominence being given to the analysis of purely clinical parameters.
LimitationsThis is an observational and single-center study that included a reduced number of patients. Patients were selected exclusively on the basis of standard indications for single-chamber implantation and a selection bias cannot be completely excluded. The stimulation mode was carefully chosen for each patient according to clinical criteria and prior to the decision to implant a leadless pacing system.
As previously mentioned, the higher cost of the Micra TPS compared with conventional pacemakers raises the question of the cost-effectiveness of the device, especially in very old patients with high mortality risk due to noncardiovascular causes. The study was not designed to evaluate the cost-effectiveness of the device in this setting, a question that certainly warrants further investigation.
CONCLUSIONSIn patients older than 90 years, implantation of the leadless intracardiac transcatheter pacemaker seems to be safe and effective, both at implantation and during follow-up, and may represent a potential reasonable alternative to conventional transvenous pacing in this population. Further studies with a larger volume of patients are warranted to confirm our observations.
CONFLICTS OF INTERESTX. Viñolas is a member of the Micra advisory Board, Medtronic. F.J. Méndez-Zurita has received honoraria from Medtronic. All other authors declare they have no conflicts of interest.
- –
The Micra TPS has been demonstrated to be safe and effective compared with conventional transvenous pacemakers. Very old patients (≥ 90 years) have a higher risk of pacemaker implantation complications. However, this population is poorly represented in large studies of the Micra TPS and the benefit of the device in this setting remains unknown.
- –
The study demonstrates that the Micra TPS is also safe and effective in the very old population (≥ 90 years).