With a prevalence of about 25% in the general population, patent foramen ovale (PFO), a special form of atrial septal defect (ASD), is a common anatomical variant. Although in most cases it remains unnoticed, it may result in tragic events such as stroke or even death. The risk of stroke appears to be higher when the PFO is associated with an atrial septal aneurysm, a Eustachian valve, or a large shunt. Although percutaneous ASD closure has been practiced since the mid-1970s and despite multiple scientific reports hinting at a substantial benefit of percutaneous PFO closure, the medical community had to wait until 2017 to obtain irrefutable scientific proof from randomized trials for the superiority of percutaneous PFO closure over conventional drug treatment.
FROM PHYSIOLOGY TO PATHOLOGYThe foramen ovale is a flap valve in the septum between the left and right atria of the heart. During fetal life, this passage allows oxygenated blood coming from the umbilical cord to bypass the lungs. Upon the first breath at birth, the foramen ovale functionally closes due to the resistance drop in the pulmonary circulation, and within the first year of life it usually seals.1 However, in 25% to 30% of cases, the foramen ovale remains open(able). This is called PFO. Problems can arise when blood containing gas bubbles or more typically solid matter (mainly blood clots) from the venous circulation shunts through the PFO from right to left into the systemic circulation. The PFO may shunt more or less frequently or even permanently. Association with an atrial septal aneurysm (ASA), a Eustachian valve, or a Chiari network renders a PFO more risky for paradoxical embolism engendering stroke as the most common clinical event.2 As in the case of atrial fibrillation (AF), PFO-mediated strokes are plausible but are usually assumed rather than proved. AF and PFO do not exclude each other as reasons for systemic embolisms like stroke. Their likelihood as culprits depends on various factors, such as chronicity and left atrial size for AF, gap size, ASA, Eustachian valve, and Chiari network for PFO, and comorbidities and age for both.
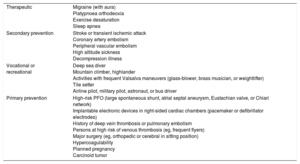
POSSIBLE CLINICAL MANIFESTATIONSThe PFO remains asymptomatic in most carriers. However, it may be responsible for several serious clinical complications, mainly through systemic embolisms. Small venous thromboembolisms have no consequences in the pulmonary circulation. They may block one of the countless small pulmonary arteries for a while before being lysed by indigenous tissue plasminogen activator but this remains clinically silent. However, such small emboli may cause strokes, myocardial infarctions, and eye problems, if they reach the systemic circulation through the PFO. Bigger clots may also cause peripheral ischemia in other territories. All systemic events are subsumed under the term paradoxical embolism. The main potential manifestations of the PFO are stroke or transient ischemic attack, embolic myocardial infarction, peripheral embolism, migraine (particularly with aura), decompression illness of divers, high altitude sickness, platypnoea orthodeoxia, exercise desaturation dyspnea, and sleep apnea.3 Ensuing indications for PFO closure are listed in Table 1.
Potential Indications for Closure of Patent Foramen Ovale
| Therapeutic | Migraine (with aura) Platypnoea orthodeoxia Exercise desaturation Sleep apnea |
| Secondary prevention | Stroke or transient ischemic attack Coronary artery embolism Peripheral vascular embolism High altitude sickness Decompression illness |
| Vocational or recreational | Deep sea diver Mountain climber, highlander Activities with frequent Valsalva maneuvers (glass-blower, brass musician, or weightlifter) Tile setter Airline pilot, military pilot, astronaut, or bus driver |
| Primary prevention | High-risk PFO (large spontaneous shunt, atrial septal aneurysm, Eustachian valve, or Chiari network) Implantable electronic devices in right-sided cardiac chambers (pacemaker or defibrillator electrodes) History of deep vein thrombosis or pulmonary embolism Persons at high risk of venous thrombosis (eg, frequent flyers) Major surgery (eg, orthopedic or cerebral in sitting position) Hypercoagulability Planned pregnancy Carcinoid tumor |
PFO, patent foramen ovale.
Percutaneous closure of the PFO is based on an intervention introduced in 1975, 2 years before the first coronary angioplasty in 1977. A double umbrella prosthesis was implanted percutaneously for the first time to close an ASD.4 Nonsurgical closure of the PFO was introduced in 1992 with the use of a similar technique.5 Since then, more than 1 million PFO closures have been performed. The intervention has become one of the most common procedures in interventional cardiology in adults. It is the safest and easiest catheter-based intervention in cardiology and may well yield the best net benefit of them all from a risk-benefit balance point of view.
RANDOMIZED DATA FAVOR PATENT FORAMEN OVALE CLOSUREThe preventive nature of the PFO closure intervention hampers its statistical analyses in randomized studies, particularly because the events it aims to prevent are rare. Thus, a large number of patients or a long follow-up are needed to achieve the statistical power required to prove a significant effect. Numerous observational comparative but nonrandomized studies have been conducted in the past comparing PFO closure with drug therapy. They have almost invariably demonstrated the usefulness of closure.6 One even showed a significant reduction in mortality.7
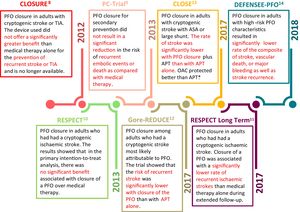
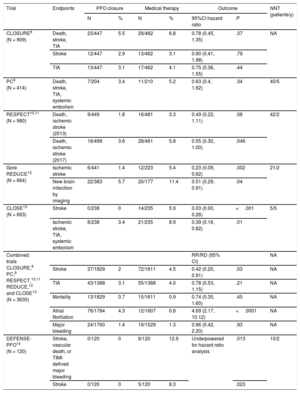
The first published randomized trials (CLOSURE,8 PC,9 and the initial report of RESPECT,10Table 2,11–14Figure 1), however, missed their primary endpoint because of a lack of statistical power (too short a follow-up, too few patients). Even though they were unable to show a statistically significant benefit of catheter-based PFO closure over conventional drug treatment, the general conclusion that PFO closure should not be performed was erroneous. Although the P values of the primary endpoints were not significant in an intention-to-treat analysis, all trials showed numerically better results with PFO closure than with medical therapy. The recurrence of ischemic vascular accidents was reduced by up to 80% in the PFO closure groups. PFO closure should have been retained as an attractive alternative to medical treatment, especially because anticoagulation continues to accumulate bleeding events at increasing pace and has an adherence problem. Neither issue exists with PFO closure. In addition, RESPECT just missed statistical significance for a treatment benefit by a hair (P=.08). Moreover, it was significant in the as-treated analysis and in predefined subgroups, such as large PFOs, PFOs associated with an ASA, or when PFO closure was compared with treatment with aspirin rather than vitamin K antagonists.
Randomized Trials of Patent Foramen Ovale Closure for Paradoxical Ischemia
| Trial | Endpoints | PFO closure | Medical therapy | Outcome | NNT (patients/y) | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | 95%CI hazard ratio | P | |||
| CLOSURE8 (N = 909) | Death, stroke, TIA | 23/447 | 5.5 | 29/462 | 6.8 | 0.78 (0.45, 1.35) | .37 | NA |
| Stroke | 12/447 | 2.9 | 13/462 | 3.1 | 0.90 (0.41, 1.98) | .79 | ||
| TIA | 13/447 | 3.1 | 17/462 | 4.1 | 0.75 (0.36, 1.55) | .44 | ||
| PC9 (N = 414) | Death, stroke, TIA, systemic embolism | 7/204 | 3.4 | 11/210 | 5.2 | 0.63 (0.4, 1.62) | .34 | 40/5 |
| RESPECT10,11 (N = 980) | Death, ischemic stroke (2013) | 9/449 | 1.8 | 16/481 | 3.3 | 0.49 (0.22, 1.11) | .08 | 42/2 |
| Death, ischemic stroke (2017) | 18/499 | 3.6 | 28/481 | 5.8 | 0.55 (0.30, 1.00) | .046 | ||
| Gore REDUCE12 (N = 664) | Ischemic stroke | 6/441 | 1.4 | 12/223 | 5.4 | 0.23 (0.09, 0.62) | .002 | 21/2 |
| New brain infarction by imaging | 22/383 | 5.7 | 20/177 | 11.4 | 0.51 (0.29, 0.91) | .04 | ||
| CLOSE13 (N = 663) | Stroke | 0/238 | 0 | 14/235 | 5.9 | 0.03 (0.00, 0.26) | <.001 | 5/5 |
| Ischemic stroke, TIA, systemic embolism | 8/238 | 3.4 | 21/235 | 8.9 | 0.39 (0.16, 0.82) | .01 | ||
| Combined: trials CLOSURE,8 PC,9 RESPECT,10,11 REDUCE,12 and CLOSE13 (N = 3630) | RR/RD (95% CI) | NA | ||||||
| Stroke | 37/1829 | 2 | 72/1611 | 4.5 | 0.42 (0.20, 0.91) | .03 | NA | |
| TIA | 43/1388 | 3.1 | 55/1388 | 4.0 | 0.78 (0.53, 1.15) | .21 | NA | |
| Mortality | 13/1829 | 0.7 | 15/1611 | 0.9 | 0.74 (0.35, 1.60) | .45 | NA | |
| Atrial fibrillation | 76/1784 | 4.3 | 12/1607 | 0.8 | 4.69 (2.17, 10.12) | <.0001 | NA | |
| Major bleeding | 24/1760 | 1.4 | 19/1529 | 1.3 | 0.96 (0.42, 2.20) | .93 | NA | |
| DEFENSE-PFO14 (N = 120) | Stroke, vascular death, or TIMI-defined major bleeding | 0/120 | 0 | 6/120 | 12.9 | Underpowered for hazard ratio analysis | .013 | 10/2 |
| Stroke | 0/120 | 0 | 5/120 | 8.3 | .023 | |||
95%CI, 95% confidence interval; NA, not available; NNT, number needed to treat; PFO, patent foramen ovale; RD, risk difference; RR, relative risk; TIA, transient ischemic attack; TIMI, Thrombolysis in Myocardial Infarction.
Timeline of most important randomized trials and their conclusions.8–13,15
ASA, atrial septal aneurysm; APT, antiplatelet therapy; OAC, oral anticoagulation; PFO, patent foramen ovale; TIA, transient ischemic attack.
*, trend.
The breakthrough finally came in 2017 with 3 additional publications on randomized trials (long-term results of RESPECT,11 Gore REDUCE,12 and CLOSE,13Table 2 and Figure 1). These reports at last provided statistically significant proof of the superiority of percutaneous PFO closure over conventional medical therapy without increased risk of serious adverse effects in the setting of secondary prevention of cryptogenic stroke, today called embolic stroke of undetermined source (ESUS).
The most important study in terms of the number of participants included was RESPECT10,11 with 980 patients randomized and a mean follow-up duration of 5.9 years. This trial compared percutaneous PFO closure associated with antiplatelet therapy vs oral anticoagulation or antiplatelet therapy alone in the setting of secondary prevention of cryptogenic stroke. Amplatzer occluders (Abbott, Plymouth, MN, United States) were used for PFO closure. The results of the long-term follow-up showed that the risk of stroke recurrence was reduced by 45% in the percutaneous PFO closure group (18/499, 3.6% in the PFO closure group vs 28/481, 5.8% in the medical therapy group) with a statically significant P value (P=.046 compared with .08 in the first analysis). The number needed to treat (NNT) calculated to 42 patients over 5 years to prevent 1 stroke. The preventive effect of PFO closure was more obvious in the subgroup of patients with an ASA or a large PFO and was significant when compared with antiplatelet treatment but was not significant when compared with oral anticoagulation.11
The second positive study was Gore REDUCE,12 conducted in 63 centers and 5 countries (United States, Canada, United Kingdom, Norway, and Sweden). A total of 664 patients with cryptogenic stroke were included with a mean follow-up of 3.2 years. PFO closure with 2 possible closure devices of the same family (Helex and Cardioform from Gore, Newark, DE, United States) associated with antiplatelet therapy was compared with antiplatelet therapy alone. The patients were randomized 2:1 to one of the 2 groups, respectively. Of the patients randomized to the antiplatelet group, 5.4% (12/223) had a stroke recurrence compared with only 1.4% (6/411) in the PFO closure group (P=.002). This corresponds to an NNT of 21 patients for prevention of 1 stroke over 2 years. Prevention by PFO closure was more pronounced in patients with at least moderate interatrial shunt.
CLOSE13 was a European trial conducted principally in France (32 centers) and Germany (2 centers) with an enrolment of a total number of 663 participants and a mean follow-up of 5.3 years. The trial compared percutaneous PFO closure associated with antiplatelet therapy (aspirin associated with clopidogrel for 3 months followed by antiplatelet monotherapy for the remainder of the trial) vs antiplatelet therapy alone in the setting of secondary prevention of cryptogenic stroke. It additionally randomized treatment with oral anticoagulation with vitamin K antagonists or nonvitamin K-dependant oral anticoagulants vs antiplatelet therapy. A key feature of this trial was that it only included participants who had a high-risk PFO, ie, one associated with an ASA or with an important right-to-left shunt. Unlike RESPECT and Gore REDUCE, in which the study closure device were defined, closure devices were left to the discretion of the operators in CLOSE. The patients were randomized 1:1:1 to 3 groups: percutaneous PFO closure group, oral anticoagulation group, and antiplatelet therapy group. Patients with a contraindication to oral anticoagulation (129 patients) were randomized to receive either PFO closure or antiplatelet therapy and those with a contraindication to PFO closure (10 patients) were randomized to receive either antiplatelet therapy or oral anticoagulation. During the follow-up period, no recurrent strokes were observed in the PFO closure group. In the medical group, 14 of the 235 (5.9%) patients had a recurrent ischemic stroke (P <.001). According to these results, the NNT is 5 patients over 5 years to prevent 1 stroke. The comparison between the anticoagulation arm and the antiplatelet arm revealed a stroke recurrence of 3/187 (1.6%) and 7/174 (4.0%), respectively, showing a trend in favor of oral anticoagulation. The trial was not powered to show a statistically significant superiority of oral anticoagulation over antiplatelet therapy. These findings are consistent with those shown by a meta-analysis of observational studies.15 Indirectly, they also confirm the finding in RESPECT that PFO closure was significantly superior to antiplatelets but not to oral anticoagulation.10,11
In 2018, the data of the prematurely interrupted DEFENSE-PFO15 trial conducted at 2 centers in South Korea were published (Table 2 and Figure 1). A mere 120 PFO patients with cryptogenic stroke and high-risk PFO (associated ASA or large shunt) were enrolled with a mean follow-up of 2.8 years. They were randomized 1:1 to 2 groups: transcatheter PFO closure group with an Amplatzer PFO Occluder or medical therapy (antiplatelet therapy or anticoagulation) alone group. Recurrence of ischemic stroke was 8.3% in the medical group vs 0% in the PFO closure group (P=.023). Because of early termination for patient safety, the study was underpowered to provide a hazard ratio but corroborated the previously published results of RESPECT, Gore REDUCE, and CLOSE. NNT in this study was only 10 patients over 2 years.
POSSIBLE COMPLICATIONS RELATED TO PERCUTANEOUS PFO CLOSUREMajor complications related to the intervention and the device, such as device embolization, cardiac perforation, free wall erosion, endocarditis, major bleeding, nitinol allergy, or death due to the procedure, are exceedingly scarce.16 AF is a rare and usually self-limiting transient complication observed following percutaneous PFO closure in 3% to 6% of patients.17 The onset of AF seems to be related to several factors such as patient age and the type of device.18 Its clinical importance is minimal and hardly modifies the benefit of PFO closure.
CONCLUSIONSRandomized data unmistakably prove the beneficial effect of PFO closure in secondary prevention of cryptogenic stroke (or in analogy of ESUS). They confirm nonrandomized comparative data. The benefit appears to be greater in the presence of a high-risk PFO (eg, large shunt or associated with ASA). The intervention is one of the safest and easiest in cardiology. Procedural complications are rare. Supraventricular arrhythmia in the first weeks are common and occasionally AF occurs but is usually self-limiting. An adaptation of national and international guidelines is expected and overdue. PFO screening should be performed in all cases of manifest systemic embolism such as stroke, even in the presence of alternative causes such as AF. PFO and cryptogenic stroke, respectively ESUS, should be defined as mutually exclusive because the PFO is a recognized cause of stroke like atherosclerosis or AF. All detected PFOs should be closed in all patients with a possibly associated index event. Based on the available data, 1 stroke event may be prevented over 2 to 20 years by closing just 10 PFOs. This number drops further with longer life expectancy, which is not unusual in these situations. PFO closure as primary prevention should also be considered in PFOs with specific risk features or in some high-risk situations. A proactive attitude is further supported by the collateral benefits of PFO closure, which are automatically present for any one of the indications.19
CONFLICTS OF INTERESTB. Meier has received speaker fees from Abbott. R. Madhkour reports no conflict of interest.