Severe calcification is present in> 50% of coronary chronic total occlusions (CTOs) undergoing percutaneous intervention. We aimed to describe the contemporary use and outcomes of plaque modification devices (PMDs) in this context.
MethodsPatients were included in the prospective, consecutive Iberian CTO registry (32 centers in Spain and Portugal), from 2015 to 2020. Comparison was performed according to the use of PMDs.
ResultsAmong 2235 patients, wire crossing was achieved in 1900 patients and PMDs were used in 134 patients (7%), requiring more than 1 PMD in 24 patients (1%). The selected PMDs were rotational atherectomy (35.1%), lithotripsy (5.2%), laser (11.2%), cutting/scoring balloons (27.6%), OPN balloons (2.9%), or a combination of PMDs (18%). PMDs were used in older patients, with greater cardiovascular burden, and higher Syntax and J-CTO scores. This greater complexity was associated with longer procedural time but similar total stent length (52 vs 57mm; P=.105). If the wire crossed, the procedural success rate was 87.2% but increased to 96.3% when PMDs were used (P=.001). Conversely, PMDs were not associated with a higher rate of procedural complications (3.7 vs 3.2%; P=.615). Despite the worse baseline profile, at 2 years of follow-up there were no differences in the survival rate (PMDs: 94.3% vs no-PMDs: 94.3%, respectively; P=.967).
ConclusionsFollowing successful wire crossing in CTOs, PMDs were used in 7% of the lesions with an increased success rate. Mid-term outcomes were comparable despite their worse baseline profile, suggesting that broader use of PMDs in this setting might have potential technical and prognostic benefits.
Keywords
Chronic total coronary occlusions (CTOs) are currently defined as a complete atherosclerotic occlusion of a vessel with Thrombolysis in Myocardial Infarction flow grade 0 with a duration greater than 3 months.1 Their prevalence ranges from 33% to 52% in patients with ischemic heart disease and their pathophysiology is still the object of thorough research. It is known that the occluded segments contain vascular tissue, lymphocytic infiltrate, fibrous tissue, atheroma, and a variable degree of calcification. Indeed, calcified atherosclerosis is the culmination of different overlapping pathological processes, probably being a cause and a consequence of cardiovascular disease, which explains why it is considered an independent cardiovascular risk factor by itself.2,3 In addition, imaging analysis has demonstrated that late stages of CTOs are associated with larger focal calcification and intraocclusion enhancement,4 ending up as larger calcium fragments that deposit in thick layers (> 3mm).5 Therefore, with these severely calcified chronic occlusions, the use of plaque modification dedicated devices (PMDs) can be helpful. There is scarce evidence supporting their use or describing the specific risks in CTO procedures. Moreover, several new PMDs have been made available in recent years.6
Hence, we aimed to describe the contemporary use of dedicated devices for severely calcified CTOs in a large cohort and compare the short- and long-term outcomes of patients who required the use of these techniques with those patients who did not.
METHODSStudy populationPatients undergoing a CTO angioplasty were prospectively and consecutively included in the Iberian CTO registry. A total of 32 sites in Spain and Portugal participated in this registry from 2015 to 2020 but only those centers who provided information on plaque modification techniques (n=17) were included in this study (). As a result, a total of 2235 patients were included; the analysis focused only on those lesions with successful wire crossing (n=1900) according to the use (n=134, 7%) or nonuse (n=1766, 93%) of PMDs. An experienced operator who had handled at least 50 CTOs and who had been previously mentored in this intervention was the first operator in all patients. Procedural success was defined as final Thrombolysis in Myocardial Infarction flow grade 3 with less than 30% residual stenosis and lack of life-threatening complications. In-hospital and follow-up outcomes were prespecified in the online database as defined in , complying with the requirements of the Law on Data Protection and accessible only to participating operators and registry coordinators. Severe calcification was defined in the database as radiopacities seen without cardiac motion before contrast injection and moderate calcification as radiopacities noted only during the cardiac cycle, but this definition varied if intravascular ultrasound demonstrated larger calcium burden than seen by angiography.3 Electrocardiographic and cardiac biomarker seriation was performed after each percutaneous coronary intervention (PCI). Clinical assessment was carried out at 1, 6, and 12 months and annually thereafter and at the end of follow-up. Angiographic follow-up was only clinically driven in patients with new symptoms, worsening ventricular function or new ischemia in noninvasive tests. The registry is endorsed by the Interventional Cardiology Association of the Spanish Society of Cardiology. The study was approved by all local ethics committees and patients provided informed consent.
DevicesPMDs can be classified into balloon (A) and nonballoon (B) techniques.7 Balloon PMDs include very high pressure noncompliant balloons (OPN NC), shear balloons (Wolverine, Boston Scientific, USA), incision balloons (AngioSculpt, Cardiva, Spain; Scoreflex, OrbusNeich, USA; NSE Alpha, Braun, Germany), and intracoronary lithotripsy balloons (Shockwave Medical Lithoplasty System, Shockwave Medical, USA). Nonballoon techniques (or debulking techniques) include rotational atherectomy (Rotablator, rotaPro, Boston Scientific, USA), orbital atherectomy (Diamondback 360, OrbusNeich,), and excimer laser atherectomy (CVX-300, Philips, UK).8 Device success was considered when the CTO lesion could be crossed and stented with Thrombolysis in Myocardial Infarction flow grade 3 with less than 30% residual stenosis. A summary of the main devices is provided in .9–21
Statistical analysisCategorical variables are presented as frequencies and comparisons between groups were performed using the chi-square or the Fisher exact test. Continuous variables are expressed as mean (± standard deviation) or median [25th-75th interquartile range] and analyzed using the Student t-test or Mann-Whitney U test, respectively. A multivariable Cox regression analysis stratified by hospital was performed to determine the predictors of 2-year mortality in the overall study population. No more than 1 variable per 10 outcome events was entered in the model to avoid overfitting. Proportional hazard assumptions were verified by the Schoenfeld residuals test and checked using log-log survival plots. The plaque modification variable, although nonsignificant, was included in the final model to provide multivariable-adjusted evidence on the effect or lack of effect of PMDs on mortality. For the final model, we calculated hazard ratios (HR) adjusted for each of the variables included, along with their 95% confidence intervals (95%CI). The robust Huber/White/Sandwich estimator was used to calculate standard errors. Goodness-of-fit for the final model was determined with the Gronnesby and Borgan test, Brier Score and C-index. All tests were 2-sided at the .05 significance level. Survival curves were estimated. The statistical analysis was performed with using IBM SPSS Statistics version 25 (IBM, USA) and R core team (2019, R Foundation for Statistical Computing, Austria).
RESULTSA total of 2235 consecutive patients from the Iberian CTO registry were prospectively included. Wire crossing was achieved in 1900 patients, and 1 PMD or more PMDs were used in 134 patients (7%) and 24 (1%), respectively.
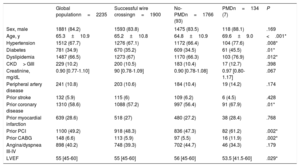
Baseline characteristics according to the use of plaque modification devicesThe main baseline characteristics are summarized in the table 1. Most patients with successful wire crossing were men (83.8%) and the median age was 65.2 years. Severe angina or dyspnea (class III or IV) were present in 40.2% and 49.1% of the patients, respectively. Most patients had prior coronary disease (57.2%) treated percutaneously (48.3%) or surgically (5.9%). In addition, 11.8% of them had a previous attempt at revascularization of a CTO.
Main baseline features
| Global populationn=2235 | Successful wire crossingn=1900 | No-PMDn=1766 (93) | PMDn=134 (7) | P | |
|---|---|---|---|---|---|
| Sex, male | 1881 (84.2) | 1593 (83.8) | 1475 (83.5) | 118 (88.1) | .169 |
| Age, y | 65.3±10.9 | 65.2±10.8 | 64.8±10.9 | 69.6±9.0 | <.001* |
| Hypertension | 1512 (67.7) | 1276 (67.1) | 1172 (66.4) | 104 (77.6) | .008* |
| Diabetes | 781 (34.9) | 670 (35.2) | 609 (34.5) | 61 (45.5) | .01* |
| Dyslipidemia | 1487 (66.5) | 1273 (67) | 1170 (66.3) | 103 (76.9) | .012* |
| CKD> GIII | 229 (10.2) | 200 (10.5) | 183 (10.4) | 17 (12.7) | .398 |
| Creatinine, mg/dL | 0.90 [0.77-1.10] | 90 [0.78-1.09] | 0.90 [0.78-1.08] | 0.97 [0.80-1.17] | .067 |
| Peripheral artery disease | 241 (10.8) | 203 (10.6) | 184 (10.4) | 19 (14.2) | .174 |
| Prior stroke | 132 (5.9) | 115 (6) | 109 (6.2) | 6 (4.5) | .428 |
| Prior coronary disease | 1310 (58.6) | 1088 (57.2) | 997 (56.4) | 91 (67.9) | .01* |
| Prior myocardial infarction | 639 (28.6) | 518 (27) | 480 (27.2) | 38 (28.4) | .768 |
| Prior PCI | 1100 (49.2) | 918 (48.3) | 836 (47.3) | 82 (61.2) | .002* |
| Prior CABG | 148 (6.6) | 113 (5.9) | 97 (5.5) | 16 (11.9) | .002* |
| Angina/dyspnea III-IV | 898 (40.2) | 748 (39.3) | 702 (44.7) | 46 (34.3) | .179 |
| LVEF | 55 [45-60] | 55 [45-60] | 56 [45-60] | 53.5 [41.5-60] | .029* |
CABG, coronary artery bypass graft; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PMD, plaque modification devices.
Values are expressed as No. (%), mean±standard deviation or median [interquartile range].
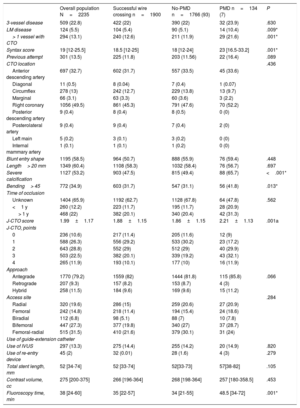
When patients were compared according to the use of plaque modification techniques, the use of dedicated devices was significantly higher in older patients (69.6±9.0 vs 64.8±10.9 years; P <.001), with higher rates of cardiovascular risk factors. In addition, they more often had prior coronary artery disease (67.9% vs 56.4%; P=.010), both surgically (11.9% vs 5.5%; P=.020) and percutaneously treated (61.2% vs 47.3%; P=.002). In particular, left main disease was more often present in the PMD group (10.4% vs 5.1%; P=.009) and this complexity was reflected by a higher Syntax score (23% vs 18%; P=.001) (table 2). Finally, the PMD group showed worse left ventricular ejection fraction (56% vs 53.5%; P=.029).
Angiographic and procedural characteristics
| Overall population N=2235 | Successful wire crossing n=1900 | No-PMD n=1766 (93) | PMD n=134 (7) | P | |
|---|---|---|---|---|---|
| 3-vessel disease | 509 (22.8) | 422 (22) | 390 (22) | 32 (23.9) | .630 |
| LM disease | 124 (5.5) | 104 (5.4) | 90 (5.1) | 14 (10.4) | .009* |
| > 1 vessel with CTO | 294 (13.1) | 240 (12.6) | 211 (11.9) | 29 (21.6) | .001* |
| Syntax score | 19 [12-25.5] | 18.5 [12-25] | 18 [12-24] | 23 [16.5-33.2] | .001* |
| Previous attempt | 301 (13.5) | 225 (11.8) | 203 (11.56) | 22 (16.4) | .089 |
| CTO location | .436 | ||||
| Anterior descending artery | 697 (32.7) | 602 (31.7) | 557 (33.5) | 45 (33.6) | |
| Diagonal | 11 (0.5) | 8 (0.04) | 7 (0.4) | 1 (0.07) | |
| Circumflex | 278 (13) | 242 (12.7) | 229 (13.8) | 13 (9.7) | |
| Marginal | 66 (3.1) | 63 (3.3) | 60 (3.6) | 3 (2.2) | |
| Right coronary | 1056 (49.5) | 861 (45.3) | 791 (47.6) | 70 (52.2) | |
| Posterior descending artery | 9 (0.4) | 8 (0.4) | 8 (0.5) | 0 (0) | |
| Posterolateral artery | 9 (0.4) | 9 (0.4) | 7 (0.4) | 2 (0) | |
| Left main | 5 (0.2) | 3 (0.1) | 3 (0.2) | 0 (0) | |
| Internal mammary artery | 1 (0.1) | 1 (0.1) | 1 (0.2) | 0 (0) | |
| Blunt entry shape | 1195 (58.5) | 964 (50.7) | 888 (55.9) | 76 (59.4) | .448 |
| Length> 20 mm | 1349 (60.4) | 1108 (58.3) | 1032 (58.4) | 76 (56.7) | .697 |
| Severe calcification | 1127 (53.2) | 903 (47.5) | 815 (49.4) | 88 (65.7) | <.001* |
| Bending> 45 | 772 (34.9) | 603 (31.7) | 547 (31.1) | 56 (41.8) | .013* |
| Time of occlusion | |||||
| Unknown | 1404 (65.9) | 1192 (62.7) | 1128 (67.8) | 64 (47.8) | .562 |
| <1 y | 260 (12.2) | 223 (11.7) | 195 (11.7) | 28 (20.9) | |
| > 1 y | 468 (22) | 382 (20.1) | 340 (20.4) | 42 (31.3) | |
| J-CTO score | 1.99±1.17 | 1.88±1.15 | 1.86±1.15 | 2.21±1.13 | .001a |
| J-CTO, points | |||||
| 0 | 236 (10.6) | 217 (11.4) | 205 (11.6) | 12 (9) | |
| 1 | 588 (26.3) | 556 (29.2) | 533 (30.2) | 23 (17.2) | |
| 2 | 643 (28.8) | 552 (29) | 512 (29) | 40 (29.9) | |
| 3 | 503 (22.5) | 382 (20.1) | 339 (19.2) | 43 (32.1) | |
| 4 | 265 (11.9) | 193 (10.1) | 177 (10) | 16 (11.9) | |
| Approach | |||||
| Antegrade | 1770 (79.2) | 1559 (82) | 1444 (81.8) | 115 (85.8) | .066 |
| Retrograde | 207 (9.3) | 157 (8.2) | 153 (8.7) | 4 (3) | |
| Hybrid | 258 (11.5) | 184 (9.6) | 169 (9.6) | 15 (11.2) | |
| Access site | .284 | ||||
| Radial | 320 (19.6) | 286 (15) | 259 (20.6) | 27 (20.9) | |
| Femoral | 242 (14.8) | 218 (11.4) | 194 (15.4) | 24 (18.6) | |
| Biradial | 112 (6.8) | 98 (5.1) | 88 (7) | 10 (7.8) | |
| Bifemoral | 447 (27.3) | 377 (19.8) | 340 (27) | 37 (28.7) | |
| Femoral-radial | 515 (31.5) | 410 (21.6) | 379 (30.1) | 31 (24) | |
| Use of guide-extension catheter | |||||
| Use of IVUS | 297 (13.3) | 275 (14.4) | 255 (14.2) | 20 (14.9) | .820 |
| Use of re-entry device | 45 (2) | 32 (0.01) | 28 (1.6) | 4 (3) | .279 |
| Total stent length, mm | 52 [34-74] | 52 [33-74] | 52[33-73] | 57[38-82] | .105 |
| Contrast volume, cc | 275 [200-375] | 266 [196-364] | 268 [198-364] | 257 [180-358.5] | .453 |
| Fluoroscopy time, min | 38 [24-60] | 35 [22-57] | 34 [21-55] | 48.5 [34-72] | .001* |
CTO, chronic total occlusion; IVUS, intravascular ultrasound; LM, left main; PMD, plaque modification devices.
Blunt entry shape: occluded segment not ending in a funnel-shape. Severe calcification: defined as radio-opacities seen without cardiac motion before contrast injection; surrounding the occlusion.
Values are expressed as No. (%), mean±standard deviation or median [interquartile range].
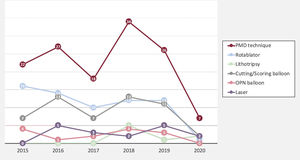
Main coronary findings during the index procedure of the overall study population and according to the use of PMD are shown in table 2. Overall, patients with successful wire crossing were more often treated with simultaneous femoral-radial access (21.6%) and via the antegrade approach (82%), which was significantly more common if PMD were used. The most frequently treated vessel was the right coronary artery (49.3%), followed by left anterior descending (31.7%) and circumflex (12.7%) arteries without differences between the PMD and non-PMD groups. J-CTO score was higher if PMD were required (2.21 vs 1.86; P=.001), mainly due to a higher degree of calcification, higher rate of previous attempts, and older CTOs, but also there was a trend to greater tortuosity of the target vessel in the PMD group. This greater complexity was associated with longer fluoroscopy time (34 vs 48.5min; P=.001) but did not have a significant impact on the contrast amount (268 vs 257cc; P=.453) or the global stented length (57 vs 52mm; P=.105). The selected PMDs were as follows: rotational atherectomy in 35.1%, lithotripsy in 5.2%, laser in 11.2%, cutting/scoring balloons in 27.6%, and OPN balloons in 2.9%. Of note, the results are reported per patient, but in certain patients each technique was used in more than 1 lesion. The temporal trend to a greater use of rotational atherectomy persisted during the study period, despite emerging alternatives as depicted in figure 1. Also reflected in this figure, there was a substantial decrease in the number of procedures occurred during the COVID-19 pandemic in 2020. To remark, rotational atherectomy was used as bailout strategy in undilatable lesions in 75.4% of the lesions, whereas in the remaining cases it was selected as the first strategy. Conversely, lithotripsy and laser were selected as the bailout strategy in all cases. More debulking strategies—including under this term rotational atherectomy and laser since both remove part of the plaque—were significantly less used in lower volume centers ().
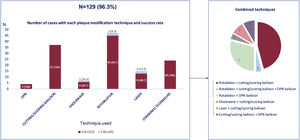
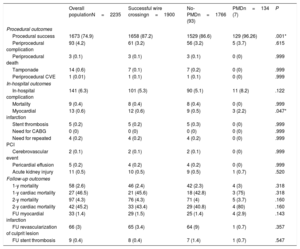
As shown in table 3, the procedural success rate was 75% in the overall population and was 87.2% in patients with successful wire crossing but increased to 96.3% when PMD were used (vs 86.6% if not used; P=.001). In particular, as shown in figure 2, the use of OPN, cutting and scoring balloons was associated with 100% success rate, whereas the use of lithotripsy had a lower success rate than other plaque modification alternatives (85.7% vs 95.7% for all others; P=.999). Rotablation and laser had presented a 95.7% and 86.7% success rate, respectively. The use of any combination of PMDs (most commonly rotablation plus another PMD, in 79.2%) was associated with a 100% technical success rate. The use of PMD was not associated with a higher rate of procedural complications (3.7% vs 3.2%; P=.615), including coronary perforation/tamponade, procedural mortality, or periprocedural stroke.
Procedural, in-hospital, and long-term outcomes
| Overall populationN=2235 | Successful wire crossingn=1900 | No-PMDn=1766 (93) | PMDn=134 (7) | P | |
|---|---|---|---|---|---|
| Procedural outcomes | |||||
| Procedural success | 1673 (74.9) | 1658 (87.2) | 1529 (86.6) | 129 (96.26) | .001* |
| Periprocedural complication | 93 (4.2) | 61 (3.2) | 56 (3.2) | 5 (3.7) | .615 |
| Periprocedural death | 3 (0.1) | 3 (0.1) | 3 (0.1) | 0 (0) | .999 |
| Tamponade | 14 (0.6) | 7 (0.1) | 7 (0.2) | 0 (0) | .999 |
| Periprocedural CVE | 1 (0.01) | 1 (0.1) | 1 (0.1) | 0 (0) | .999 |
| In-hospital outcomes | |||||
| In-hospital complication | 141 (6.3) | 101 (5.3) | 90 (5.1) | 11 (8.2) | .122 |
| Mortality | 9 (0.4) | 8 (0.4) | 8 (0.4) | 0 (0) | .999 |
| Myocardial infarction | 13 (0.6) | 12 (0.6) | 9 (0.5) | 3 (2.2) | .047* |
| Stent thrombosis | 5 (0.2) | 5 (0.2) | 5 (0.3) | 0 (0) | .999 |
| Need for CABG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .999 |
| Need for repeated PCI | 4 (0.2) | 4 (0.2) | 4 (0.2) | 0 (0) | .999 |
| Cerebrovascular event | 2 (0.1) | 2 (0.1) | 2 (0.1) | 0 (0) | .999 |
| Pericardial effusion | 5 (0.2) | 4 (0.2) | 4 (0.2) | 0 (0) | .999 |
| Acute kidney injury | 11 (0.5) | 10 (0.5) | 9 (0.5) | 1 (0.7) | .520 |
| Follow-up outcomes | |||||
| 1-y mortality | 58 (2.6) | 46 (2.4) | 42 (2.3) | 4 (3) | .318 |
| 1-y cardiac mortality | 27 (46.5) | 21 (45.6) | 18 (42.8) | 3 (75) | .318 |
| 2-y mortality | 97 (4.3) | 76 (4.3) | 71 (4) | 5 (3.7) | .160 |
| 2-y cardiac mortality | 42 (45.2) | 33 (43.4) | 29 (40.8) | 4 (80) | .160 |
| FU myocardial infarction | 33 (1.4) | 29 (1.5) | 25 (1.4) | 4 (2.9) | .143 |
| FU revascularization of culprit lesion | 66 (3) | 65 (3.4) | 64 (9) | 1 (0.7) | .357 |
| FU stent thrombosis | 9 (0.4) | 8 (0.4) | 7 (1.4) | 1 (0.7) | .547 |
CABG, coronary artery bypass graft; CVE, cerebrovascular event; FU, follow-up; PCI, percutaneous coronary intervention; PMD, plaque modification devices.
Values are expressed as No. (%).
Complications and mortality are shown in table 3. In-hospital complications were comparable irrespective of the use of PMDs, need for coronary artery bypass graft surgery (0%) or new target vessel PCI (0.2%), cerebrovascular events (0.1%), cardiac tamponade (0.6%), contrast-induced nephropathy (0.5%), or in-hospital mortality (0.4%). Only periprocedural myocardial infarction was more likely following PMD use (2.2% vs 0.5%, P=.047), but was not associated with a difference in the rate of acute stent thrombosis (0% vs 0.3%, P=.999).
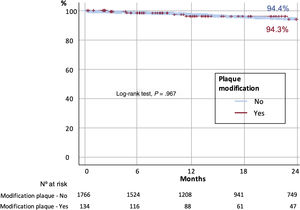
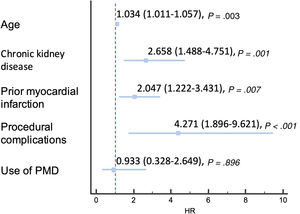
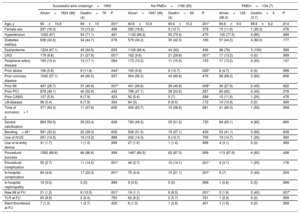
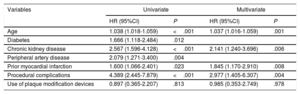
At 2 years of follow-up, there were no differences in survival according to the use or nonuse of PMD (96.3% vs 96%, respectively; P=.160) (figure 3), and overall mortality was 4.3%, mainly due to noncardiovascular causes (56.6%). The main factors associated with higher overall mortality are summarized in table 4 and independent predictors of mortality are depicted in figure 4 and table 5, including age, chronic kidney disease, prior myocardial infarction, and procedural complications, but not the use of any of the PMDs included in this study or the institution where it was performed. Moreover, despite the worse baseline profile of patients requiring PMDs, their mortality was comparable.
Predictors of 2-year mortality
| Successful wire crossingn=1900 | No-PMDn=1766 (93) | PMDn=134 (7) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Aliven=1824 (96) | Deathn=76 (4) | P | Aliven=1697 (96) | Deathn=69 (4) | P | Aliven=129 (96.3) | Deathn=5 (3.7) | P | |
| Age, y | 65±10.8 | 69±10 | .001* | 64.6±10.9 | 69.6±10.2 | .001* | 69.6±9.0 | 68.6±9.2 | .814 |
| Female sex | 297 (16.3) | 10 (13.2) | .468 | 282 (16.6) | 9 (12.7) | .378 | 15 (11.6) | 1 (20.0) | .476 |
| Hypertension | 1222 (67) | 54 (71.1) | .461 | 1122 (66.2) | 50 (70.4) | .470 | 100 (77.5) | 4 (80) | .999 |
| Diabetes mellitus | 636 (34.9) | 34 (44.7) | .078 | 579 (34.2) | 30 (42.3) | .160 | 57 (44.2) | 4 (80.0) | .177 |
| Dyslipidemia | 1224 (67.1) | 49 (64.5) | .633 | 1126 (66.4) | 44 (62) | .436 | 98 (76) | 5 (100) | .589 |
| CKD | 179 (9.8) | 21 (27.6) | .001* | 162 (9.6) | 21 (29.6) | .001* | 17 (13.2) | 0 (0) | .999 |
| Peripheral artery disease | 190 (10.4) | 13 (17.1) | .064 | 173 (10.2) | 11 (15.5) | .153 | 17 (13.2) | 2 (40) | .147 |
| Prior stroke | 106 (5.8) | 9 (11.8) | .044* | 100 (5.9) | 9 (12.7) | .020* | 6 (4.7) | 0 (0) | .999 |
| Prior coronary disease | 1042 (57.1) | 46 (60.5) | .557 | 954 (56.3) | 43 (60.6) | .476 | 88 (68.2) | 3 (60) | .656 |
| Prior MI | 487 (26.7) | 31 (40.8) | .007* | 451 (26.6) | 29 (40.8) | .008* | 36 (27.9) | 2 (40) | .622 |
| Prior PCI | 878 (48.1) | 40 (52.6) | .442 | 798 (47.1) | 38 (53.5) | .287 | 80 (62) | 2 (40) | .376 |
| Prior CABG | 107 (5.9) | 6 (7.9) | .453 | 92 (5.4) | 5 (7) | .589 | 15 (11.6) | 1 (20) | .476 |
| LM disease | 98 (5.4) | 6 (7.9) | .304 | 84 (5) | 6 (8.5) | .172 | 14 (10.9) | 0 (0) | .999 |
| Time of occlusion> 1 y | 371 (63.3) | 11 (57.9) | .630 | 330 (63.7) | 10 (58.8) | .681 | 41 (60.3) | 1 (50) | .999 |
| Severe calcification | 864 (50.5) | 39 (53.4) | .628 | 780 (49.3) | 35 (51.5) | .730 | 84 (65.1) | 4 (80) | .660 |
| Bending> 45° | 581 (32.2) | 22 (29.3) | .602 | 528 (31.5) | 19 (27.1) | .439 | 53 (41.1) | 3 (60) | .649 |
| Use of IVUS | 261 (14.3) | 10 (13.2) | .868 | 242 (14.3) | 9 (12.7) | .705 | 19 (14.7) | 1 (20) | .560 |
| Use of re-entry device | 31 (1.7) | 1 (1.3) | .999 | 27 (1.6) | 1 (1.4) | .999 | 4 (3.1) | 0 (0) | .999 |
| Procedural success | 1580 (86.6) | 66 (86.8) | .999 | 1467 (86.5) | 62 (87.3) | .999 | 113 (87.6) | 4 (80) | .498 |
| Procedural complication | 50 (2.7) | 11 (14.5) | .001* | 46 (2.7) | 10 (14.1) | .001* | 4 (3.1) | 1 (20) | .176 |
| In-hospital complication | 84 (4.6) | 17 (22.4) | .001* | 75 (4.4) | 15 (21.1) | .001* | 9 (7) | 2 (40) | .054 |
| In-hospital nephropathy | 10 (0.5) | 0 (0) | .999 | 9 (0.5) | 0 (0) | .999 | 1 (0.8) | 0 (0) | .999 |
| New MI at FU | 21 (1.2) | 8 (10.5) | .001* | 19 (1.1) | 6 (8.5) | .001* | 2 (1.6) | 2 (40) | .007* |
| TLR at FU | 63 (8.9) | 2 (5.4) | .763 | 62 (9.2) | 2 (5.7) | .761 | 1 (2.9) | 0 (0) | .999 |
| Stent thrombosis at FU | 7 (1.3) | 1 (2.7) | .420 | 6 (1.3) | 1 (2.8) | .401 | 1 (1.9) | 0 (0) | .999 |
CABG, coronary artery bypass graft; CKD, chronic kidney disease; CTO, chronic total occlusion; FU, follow-up; IVUS, intravascular ultrasound; LM, left main; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PMD, plaque modification devices; TLR, target lesion revascularization.
Values are expressed as No. (%), or median [interquartile range].
Independent predictors of mortality in successful CTO wire crossing
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.038 (1.018-1.059) | <.001 | 1.037 (1.016-1.059) | .001 |
| Diabetes | 1.666 (1.118-2.484) | .012 | ||
| Chronic kidney disease | 2.567 (1.596-4.128) | <.001 | 2.141 (1.240-3.696) | .006 |
| Peripheral artery disease | 2.079 (1.271-3.400) | .004 | ||
| Prior myocardial infarction | 1.600 (1.066-2.401) | .023 | 1.845 (1.170-2.910) | .008 |
| Procedural complications | 4.389 (2.445-7.879) | <.001 | 2.977 (1.405-6.307) | .004 |
| Use of plaque modification devices | 0.897 (0.365-2.207) | .813 | 0.985 (0.353-2.749) | .978 |
95%CI, 95% confidence interval; HR, hazard ratio. Groennesby and Borgan test, P=.168. C-index 0.672 (0.612, 0.732). Brier score: 0.028 (0.027, 0.029).
Percutaneous treatment of CTOs is technically demanding, and the success rate is still suboptimal worldwide. Intraplaque calcification is a well-known predictor of technical failure22,23 mainly due to uncrossable or undilatable lesions that can affect up to 6% and 12%24,25 of lesions, respectively, but also due to higher rate of complications (∼8%)—mainly perforations—that might require interruption of the procedure. This is important, given that moderate-severe calcification of CTOs affects up to 58% of persons deemed candidates for PCI.26 In this subanalysis of the Iberian CTO registry, we performed a thorough description of the main current tools specifically dedicated to the management of severe calcification and the main findings were: a) the most commonly used technique was rotational atherectomy, despite the growing range of alternatives, and the success rate following PMDs reached 95% when only 1 device was used and 100% when 2 devices were used, suggesting a synergic behavior of these technologies; b) patients who needed modification plaque techniques had a significantly greater cardiovascular risk, greater calcification in the occluded segment, and greater J-CTO score; c) despite this, the complication and mortality rates during complex CTO procedures was very low and there were no differences in 2-year survival rates between the modification and nonmodification plaque groups, suggesting that the use of these techniques might be helpful for buffering the increased atherosclerotic burden in this subset of patients.
Baseline risk and technical success in patients treated with PMDIt is well known that PMD are often used in older patients with a higher rate of cardiovascular risk factors and, accordingly, more complex coronary disease.2,3 However, the prognostic impact at mid-term of these technologies is less clear. The greatest evidence has been obtained for the most commonly used technique, rotational atherectomy.27 However, in recent years, the use of lithotripsy and excimer laser coronary angioplasty as new alternatives has progressively increased in clinical practice; in the setting of CTOs, their lower use could be explained the scarce evidence but also by their limited availability in some centers.12,19 However, these new techniques were safe and effective according to our results, particularly if used in combination, suggesting a similar behavior as that reported for non-CTO lesions in the DISRUPT CAD II, the STRATAS trial, or the LEONARDO trial, all reporting success rates above 90%.13,21,28
Importantly, in this study we compared the acute and mid-term outcomes according to the use of PMDs after excluding patients in whom the wire did not cross the CTO and, nevertheless, a significant and greater success rate (96.3% vs 86.6%) was observed for the PMD cohort; however, the use of rotational atherectomy and laser are often reserved for balloon-undilatable lesions,20,29 whereas the other PMDs are often used to improve angiographic results in lesions can also be crossed and dilated with standard balloons and stents. In this last setting, randomized analysis might shed some light on the best strategy; conversely, ... for undilatable lesions, future studies randomizing to PMDs or no-PMDs are unlikely.
Long-term outcomes in patients with calcified CTOs treated with PMDAt longer term, it is reasonable to wonder whether treating highly calcified CTOs might be related to a higher target vessel failure rate, as suggested by some authors.22 However, in our study, the 2-year outcomes were favorable compared with patients with less complex disease, suggesting that a greater use of these technologies might be potentially useful for better long-term patency of percutaneously treated CTOs if a moderate or severe degree of calcification is present. Intravascular imaging analysis was available in 37% of the study population, suggesting that moderate-severe calcification is present in> 50% of the CTOs and that stent underexpansion is often detected by these techniques (∼3%) but not by angiography.30 The complementary effect of intravascular imaging and PMDs might be crucial for the improvement of long-term outcomes in patients with CTOs undergoing percutaneous recanalization; in this subset, intravascular ultrasound was used in 56% of the patients during the index procedure (P=.001 compared with its use in patients without plaque modification strategies), highlighting the complexity of patients requiring PMDs, but also potentially influencing their adequate mid-term outcomes reported in this study. Although this study was underpowered to compare different PMDs, it is evident that there are “less debulking” and “more debulking” techniques, including in this last group orbital atherectomy, rotational atherectomy, and laser. The hypothesis that more debulking strategies might provide better long-term outcomes remains unproved, but the fact that these strategies were significantly less used in lower volume centers () suggests that the degree of operator proficiency required might be greater to use them to avoid an excessive complication rate that might otherwise counterbalance this potential benefit in terms of recanalization durability. Indeed, among the independent predictors of mortality and as opposed to the nonmodifiable risk factors (age, chronic kidney disease, and peripheral artery disease), periprocedural complications are a potential factor that can be improved to reduce mortality.31,32
LimitationsThe main limitations of this study, inherent to the retrospective nature of registry-based studies, are the heterogeneity of PMD techniques (number of operators, operatorś experience-based criteria of PMD use, and cath-lab availability of each PMD), a certain bias due to the lack of an independent core-lab analysis to evaluate angiographic and clinical events and the small plaque modification cohort. Despite this heterogeneity of the included techniques the common underlying disease in which they were used, more than specific technical aspects, was taken into consideration for the present analysis and the evaluation of outcomes at longer term follow-up was thoroughly reviewed.
CONCLUSIONSPMDs for severely calcified CTOs were used in 6% of the patients with an optimal success rate, particularly when several of these techniques were combined. Mid-term outcomes were comparable to those in patients not requiring plaque modification, despite their greater baseline risk, suggesting that broader use of PMDs in this setting might have potential technical and prognostic benefits.
FUNDINGNone to declare
AUTHORS’ CONTRIBUTIONSI. J. Amat-Santos and J.A. Fernández-Díaz coordinated the registry. J.R. Delgado-Arana and I. J. Amat-Santos designed the project and were in charge of manuscript writing and submission. J. R. Rumoroso, A. Regueiro, J. Martín-Moreiras, G. Miñana, M. Mohandes, M. Pan, P. Salinas, J. Caballero-Borrego, J. A. Fernández-Díaz, A. Jurado, J. Lacunza, B. Vaquerizo, F. Rivero, J. Abellán, J. Rondán, A. Gómez Menchero, S. Santos-Martínez, A. Subinas, V. Arévalos, A. Diego Nieto, J. Sanchis, S. Rojas, S. Ojeda, N. Gonzalo, M. López-Pérez, J. Goicolea, M. Sádaba, M. Sabaté, and J. C. Núñez García performed the interventions, collected the information prospectively, carried out the follow-up, and revised, and approved the final version of the manuscript. I. Gómez-Salvador performed the statistical analysis and full paper review and approval.
- -
Severe calcification is present in> 50% of CTOs undergoing percutaneous intervention.
- -
Several new PMDs have entered the market in recent years and, in addition to those already in use, have spread rapidly.
- -
However, little is known about the rate of use and outcomes in the context of severely calcified CTOs.
- -
PMDs for severely calcified CTOs were used in 7% of the patients with an optimal success rate, particularly when several of these techniques were combined.
- -
Mid-term outcomes were similar to those in patients not requiring plaque modification, despite their greater baseline risk, suggesting that broader use of PMDs in this setting might have potential technical and prognostic benefits.
A. Regueiro is consultant for Boston Scientific; iVascular; Terumo. A. Jurado is proctor for Spectranetics. M. Sabaté is consultant for Abbott Vascular and iVascular outside the submitted work. J. R. Rumoroso and I. J. Amat-Santos are proctors for Boston Scientific.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.06.011