Keywords
INTRODUCTION
Heart failure is characterized by the notable activation of different circulating neurohormone systems.1 The level of this activation is related to functional capacity,2 the degree of ventricular dysfunction, and mortality.3-5
Raised concentrations of endothelin-1 (ET1), and of its precursor big endothelin-1 (big ET-1), provide an important, independent indicator of heart failure in congestive heart disease,6 and are related to pulmonary hypertension in patients with this problem,6,7 the severity of their overall condition, and their prognosis.8,9 The endothelins are a family of proteins (ET-1, ET2, ET-3, and ET-4) consisting of 21 amino acids. They are produced by the endothelial cells,10 the smooth muscle cells of the blood vessels, and the cardiac myocytes,11 and exert effects on vasomotor tone, cell proliferation, and the production of modulatory hormones.11 Endothelin-1 (ET-1) is formed from its biological precursor big ET-1, a 38 amino acid-long peptide that, after its synthesis in the cytoplasm, is cleaved by endothelin conversion enzyme to yield active ET-1 (amino acids 1-21) and a C terminal fragment (amino acids 22-38).10 The physiological importance of this conversion of big ET-1 into ET-1 is that the vasoconstrictive power of the final product is 140 fold greater.12
Another family of neurohormones of great pathophysiological importance in the diagnosis of heart failure, as well as in the risk stratification and follow-up of patients treated for this problem, is that of the natriuretic peptides.13,14 Four types of ligand (ANP, BNP, CNP, and DNP) and 3 types of receptor (NPR-A, NPR-B, and NPR-C) have been described for this family. Owing to its rapid and specific expression in heart failure, as well as its correlation with wall stress, brain natriuretic peptide (BNP) is now recognized as an excellent marker of ventricular dysfunction.15,16 This peptide is mainly synthesized in the heart as a prohormone (proBNP), which is hydrolyzed to produce the biologically active form BNP plus an N terminal fragment (NT-proBNP). The mean half-life and blood values of the latter are greater than those of BNP, and the molecule is highly specific for the diagnosis of heart failure.17,18
A close relationship exists between ET-1 and the natriuretic peptides of the cardiovascular system. Several studies have shown ET-1 to be a potent stimulator of the synthesis and release of these peptides in cardiac tissues.19,20 It has also been suggested that part of the vasodilatory action of these natriuretic peptides might be due to a reduction in the basal production of ET-1.11,21 The hypothesis has been advanced that a feedback mechanism exists between them, contributing to the regulation of vascular tone.22,23
Given that ET-1 and big ET-1 are found in equimolar concentrations in the plasma,6 that big ET-1 has a longer half-life and is cleared more slowly, and that increased plasma levels of ET-1 in patients with heart failure are owed mainly to an increase in the concentration of big ET-1,8 the aim of this work was to compare the plasma levels of big ET-1 and NT-proBNP in a cohort of such patients, and to analyze the relationships between big ET-1 levels and the ventricular function parameters that might be affected by such neurohormonal activation.
PATIENTS AND METHODS
Patients
The study population included 103 patients diagnosed with heart failure, all recruited from the cardiology departments of 6 hospitals in the Comunidad Valenciana (the autonomous region of Valencia, Spain). All patients completed a specific questionnaire and underwent a physical examination, an electrocardiogram, a chest x-ray, a Doppler echocardiographic study, and blood extraction for hematological and biochemical analyses. Given the difficulties experienced in taking Doppler readings, patients in atrial fibrillation, with acute coronary syndromes,24 chronic or acute liver disease,25 chronic infections,26 kidney disease,27 or chronic obstructive pulmonary disease28 were excluded from the study. Some 11.6% of the possible patients examined at the different hospitals were excluded for these reasons. The causes of heart failure in the studied patients were ischemic heart disease (n=42; 41%), dilated cardiomyopathy (n=43; 43%), hypertensive cardiomyopathy (n=13; 13%) and valve disease (n=3; 3%). Of the 103 patients studied, 44 (43%) were hypertensive and 39 (38%) diabetic. All were functionally classified according to the criteria of the New York Heart Association and received medical treatment according to the American Heart Association29 and the European Society of Cardiology.30 Some 79% received diuretics, 74% received inhibitors of angiotensin converting enzyme, 51% were treated with beta-blockers, 46% with anti-aldosterone agents, 33% with digitalic drugs, 15% with calcium antagonists, and 14% with angiotensin II receptor antagonists. All patients received stable medical treatment for 1 month before commencement of the study. Table 1 shows the main clinical characteristics of the patients.
The study was approved by the appropriate review boards and ethics committees, and performed in accordance with good clinical practice guidelines and ethical standards for human experiments set out in the Declaration of Helsinki. All patients gave their informed, written consent to be included in the study.
Echocardiographic Study
Standard echographic systems equipped with 2.5 MHz transducers (employed routinely by all 6 hospitals involved in the study) were used to examine all patients. Echocardiographic results and Doppler traces were recorded on videotape for later, centralized analysis (blind to all other results) using the Eco-Dat computer system (Eco-Dat; Software de Medicina S.A.). Parasternal and apical planes were obtained with the patient laying on his/her left side. Measurements were taken during respiratory apnea; means for 4 cardiac cycles were calculated.
The mitral flow propagation velocity (Vp) was determined as previously decribed,31 the ejection fraction (EF) was determined using the area-length method,32 and the atrioventricular plane displacement (AVPD) was determined in M mode from a 2-dimensional apical projection (2 and 4 chamber views).33 The mean of the septal, lateral, posterior, and anterior regions was calculated. In the determination of AVPD, interobserver and intraobserver variability was calculated for a series of 50 patients; the results were 4.6±4.9% and 2.9±4.2%, respectively. For Vp determinations these variations were 8±8% and 7.2±8%, respectively. Variabilities were expressed as the absolute difference divided by the mean value of the means.
Determination of NT-proBNP, big Endothelin-1 and Aldosterone
Blood samples were taken by venipuncture (EDTA-tubes) with the patient having rested in a supine position for 30 min. Individual samples were separated by centrifugation at 3000 rpm for 10 min at room temperature and the plasma then frozen at 80ºC for transport to the analytical laboratory.
Plasma NT-proBNP was determined blind and in duplicate by electrochemiluminescent immunoanalysis (ECLIA) (Elecsys® proBNP, Roche Diagnostics).34 Results were expressed in pg/mL.
Plasma big ET-1 was determined by enzymatic immunoanalysis (ELISA) (Biomedica Gruppe, Germany); the results were expressed in fmol/mL. The interassay and intra-assay variability was 6.1% and 3.9%, respectively.
Plasma aldosterone was determined by radioimmunoanalysis and the results expressed in pg/mL. The interassay and intra-assay variability was 3.8% and 3%, respectively.
Statistical Analysis
Data for normally distributed variables were expressed as means ± standard deviation (SD), as medians and interquartile ranges for non-normally distributed variables, and as frequencies for categorical variables. The normality of each variable was examined using the Kolmogorov-Smirnov test. The results for neurohormones with a non-normal distribution were log-transformed for statistical analysis. Pearson correlation coefficients were used to describe the linear association between the values for big ET-1, NT-proBNP, and the different echocardiographic parameters. ANOVA (for multiple comparisons) was used to compare the mean values for NT-proBNP, EF, Vp, and AVPD between the different groups defined by the ET-1 quartiles. All analyses were performed using the Software Package for the Social Sciences (SPSS/PC) v. 10.1 (SPSS Inc. Chicago, Illinois). Significance was set at P<.05.
RESULTS
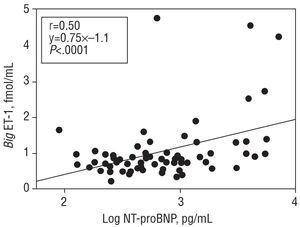
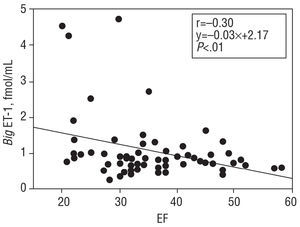
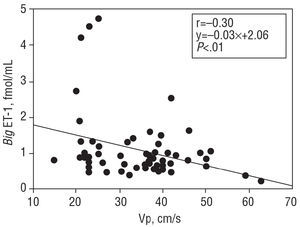
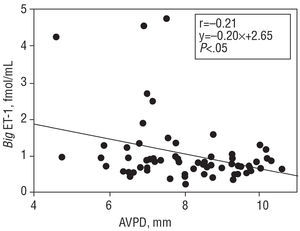
For the patient population as a whole, the mean big ET-1 concentration was 1.03±0.75 fmol/mL, NT-proBNP was 619 (3071.328) pg/mL, and aldosterone 168±102 pg/mL. Big ET-1 levels correlated positively with those of NT-proBNP (r=0.50; P<.0001) (Figure 1). When the correlation between the latter variables was examined in the subgroup of patients with dilated cardiomyopathy, a value of r=0.6 (P<.0001) was obtained. When big ET-1 values were examined in relation to ventricular function parameters, a significant inverse relationship was obtained with EF (r=-0.30; P<.01) (Figure 2), Vp (r=-0.30; P<.01) (Figure 3), and AVPD (r=-0.21; P<.05) (Figure 4).
Figure 1. Direct correlation between plasma big endothelin-1 (big ET-1) concentrations and the logarithm of plasma N-terminal pro-brain natriuretic peptide (log NT-proBNP).
Figure 2. Inverse correlation between plasma big endothelin-1 (big ET-1) concentration, and ejection fraction (EF).
Figure 3. Inverse correlation between plasma big endothelin-1 (big ET-1) concentration and mitral flow propagation velocity (Vp).
Figure 4. Inverse correlation between plasma big endothelin-1 (big ET-1) concentration and atrioventricular plane displacement (AVPD).
When the big ET-1 values were divided into quartiles (Q1 0.47±0.13 fmol/mL; Q2 0.74±0.06 fmol/mL; Q3 0.94±0.05 fmol/mL; Q4 1.84±1.0 fmol/mL) and compared to the mean values for NT-proBNP in each subgroup (Q1 442 [267-74] pg/mL; Q2 505 [272-955] pg/mL; Q3 531 [238-1.754] pg/mL; Q4 1.394 [545-4.036] pg/mL), the correlation was significant (P<.0001) (Figure 1). In addition, when the mean values for the ventricular function parameters in each big ET-1 quartile were compared, the following results are obtained: for EF (Q1 39±10; Q2 40±10; Q3 35±9; Q4 33±11), P<.01; for Vp (Q1 39±9; Q2 38±13; Q3 37±13; Q4 34±8), P<.05, and for AVPD (Q1 8.1±1.1; Q2 8.7±2.0; Q3 7.8±1.4; Q4 7.4±2.1), P<.05.
No significant correlation was found between big ET-1 and aldosterone levels, nor were any significant differences found when comparing the mean aldosterone values in the different big ET-1 quartiles.
DISCUSSION
Though some studies relate ET-1 values with those of the natriuretic peptides35 and with ventricular function in patients with heart failure, we decided to measure the levels of big ET-1 since these might better reflect the activities of the big ET-1/ET-1 complex. It has been shown that big ET-1 has a longer half-life and slower clearance than ET-1. The increased plasma levels of ET-1 in heart failure patients is mainly due to an increase in big ET-1 concentrations since ET-1, like all biologically highly active peptides, is cleared rapidly from the organism and its paracrine activity is not reflected by its blood concentration.8
A close relationship exists between ET-1 and natriuretic peptides in the cardiovascular system. Endothelin-1 is a potent stimulator of the synthesis and release of these peptides in cardiac tissues,19,20 and it has been suggested that part of the vasodilatory action of the natriuretic peptides might be due to a reduction in the basal production of ET-1.11,21 A feedback mechanism between them that helps regulate vascular tone has been hypothesized.22,23 Given that the synthesis of ET-1 is closely related to big ET-1 levels,8 this inhibitory effect should be highlighted when correlating levels of this precursor with those of NT-proBNP. However, our results show a strong positive correlation between both (Figure 1), a finding in agreement with earlier studies that correlated ANP levels with those of big ET-1.6,9 These results show the importance of the pressure-volume changes in which both systems are involved, and although their 2 agents have opposite effects on some hemodynamic variables, the levels of both can be used as prognostic markers in patients with heart failure.9,18
The present results also show that plasma big ET-1 concentrations are inversely related to the EF, Vp and AVPD. These inverse correlations are shown when the big ET-1 values are divided into quartiles and then correlated with values for NT-proBNP. A more noticeable deterioration in ventricular function is seen in the group of patients with the highest big ET-1 levels.9 The cause of this inverse relationship between ventricular function and big ET-1 concentration is unclear, but it could be partly due to the effect of high ET-1 concentrations on kidney function and the secretion of aldosterone, leading to the retention of salt and water and a subsequent increase in intravascular volume.36 Endothelin-1 also stimulates sympathetic nervous activity and arterial vasoconstriction,36 thus increasing systemic vascular resistance. Several studies have described the inverse relationship between big ET-1 concentration and EF,6,9 but none have related the concentration of this peptide to Vp or AVPD, two variables of great interest in patients with heart failure.32 The Vp is a diastolic function variable relatively independent of preload31 that reflects the state of ventricular relaxation and shows a linear behavior unlike other diastolic function variables whose values are reduced as function deteriorates.31 This characteristic facilitates its comparison with other biochemical variables with similar behavior. In addition, the relationship between the NT-proBNP values and ventricular function is similar, i.e., high concentrations of natriuretic peptide are accompanied by low EF and AVPD values.37
It is known that neurohormone concentrations are influenced by the medication given to patients, and it has recently been shown that exogenous nesiritide reduces plasma ET-1 concentrations in patients with heart failure.38 However, there have been few studies on the influence of medication on the levels of its precursor big ET-1.39 In any event, the results obtained in the present work are in agreement with those of earlier papers in which comparisons were made with plasma ANP levels.6,9
Limitations of the Study
The majority of the present patients fell into a moderate functional class (NYHA II); the most deteriorated classes were not represented. Even so, there was a high percentage of patients in class III (18.5%), which sugg ests the present group of patients is sufficiently representative. This, plus the treatment provided, explains why the concentrations of big ET-1 recorded were not very high compared to those obtained in other studies6,9 (although they are comparable to those reported in yet others35).
With respect to the echocardiographic study, the use of different Doppler apparatuses could have introduced variability in terms of image acquisition. However, the cardiologists who undertook these analyses perform such work routinely in their hospitals and have participated in previous studies. In addition, a video was sent to each participating unit to show how to proceed in this analysis, thus favoring uniformity in the acquisition of images.
It has been reported that the measurement of ventricular function is more precise when magnetic resonance is used.40 However, the fact that the present analyses were undertaken centrally by a cardiologist experienced in Doppler techniques, plus the low variability obtained, suggest the results are valid.
CONCLUSIONS
This multicentre study shows the correlation between plasma big ET-1 and NT-proBNP levels in a group of patients with heart failure. Increased big ET-1 concentrations are associated with increased ventricular dysfunction. These findings assist in understanding the complex relationship between the neurohormone systems in heart failure, their influence on changes in ventricular function, and the survival of heart failure patients.
This projected was funded by the Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III, Project FIS 01/0943.
Correspondence: Dr. J. M. Rivera Otero.
Servicio de Cardiología. Centro de Investigación Hospital La Fe.
José María Haro, 59, puerta 59. 46022 Valencia. España.
E-mail: rivera_jmi@gva.es