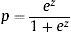Infective endocarditis (IE) is a complex disease with high in-hospital mortality. Prognostic assessment is essential to select the most appropriate therapeutic approach; however, international IE guidelines do not provide objective assessment of the individual risk in each patient. We aimed to design a predictive model of in-hospital mortality in left-sided IE combining the prognostic variables proposed by the European guidelines.
MethodsTwo prospective cohorts of consecutive patients with left-sided IE were used. Cohort 1 (n=1002) was randomized in a 2:1 ratio to obtain 2 samples: an adjustment sample to derive the model (n=688), and a validation sample for internal validation (n=314). Cohort 2 (n=133) was used for external validation.
ResultsThe model included age, prosthetic valve IE, comorbidities, heart failure, renal failure, septic shock, Staphylococcus aureus, fungi, periannular complications, ventricular dysfunction, and vegetations as independent predictors of in-hospital mortality. The model showed good discrimination (area under the ROC curve=0.855; 95%CI, 0.825-0.885) and calibration (P value in Hosmer-Lemeshow test=0.409), which were ratified in the internal (area under the ROC curve=0.823; 95%CI, 0.774-0.873) and external validations (area under the ROC curve=0.753; 95%CI, 0.659-0.847). For the internal validation sample (observed mortality: 29.9%) the model predicted an in-hospital mortality of 30.7% (95%CI, 27.7-33.7), and for the external validation cohort (observed mortality: 27.1%) the value was 26.4% (95%CI, 22.2-30.5).
ConclusionsA predictive model of in-hospital mortality in left-sided IE based on the prognostic variables proposed by the European Society of Cardiology IE guidelines has high discriminatory ability.
Keywords
Left-sided infective endocarditis (LSIE) is a rare disease with high mortality, ranging from 15% to 40%.1–4 Prognosis may be improved by some recent advances, such as new indications for imaging techniques, potent new antibiotics, and early surgery. However, although adjusted mortality may have decreased, absolute mortality remains steady.5–7
With such a bleak prognosis, early identification of patients with poor short-term outcome is crucial and could have influence the natural history of the disease. Although the European guidelines for infective endocarditis (IE) insist on prognostic assessment, they only provide a list of 19 individual variables associated with poor outcome, and evidence for some of these variables is weak. The American guidelines do not provide any recommendations in this regard.8,9
The prognostic factors provided by the European guidelines are divided into 4 groups: 4 variables are related to patient characteristics (older age, prosthetic valve IE, diabetes mellitus, and comorbidity); 5 to clinical complications (heart failure, renal failure, moderate area of ischemic stroke, brain hemorrhage, and septic shock); 3 to the causative microorganism (Staphylococcus aureus, fungi, and Gram-negative bacilli); and 7 are echocardiographic findings (periannular complications, severe left-sided valve regurgitation, low left ventricular ejection fraction, pulmonary hypertension, large vegetation, severe prosthetic valve dysfunction, and premature mitral valve closure, and other signs of elevated diastolic pressures). For some of these variables, there is almost no evidence supporting their prognostic value. In addition, the prognostic impact of each variable is not weighted and some of them undoubtedly carry a worse prognosis than others.
We aimed to derive and validate a model to predict the short-term outcome of patients with LSIE based on these variables by using a large population of patients with LSIE.
METHODSStudy populationTwo prospective cohorts of consecutive patients with LSIE from 4 tertiary university hospitals were used in this study. The first cohort (cohort 1) included all patients consecutively diagnosed with definite LSIE between 2000 and 2017 from 3 hospitals and was used to derive and internally validate the predictive model. The second cohort (cohort 2), used for the external validation, included all patients with a final diagnosis of LSIE between 2012 and 2017 admitted to another hospital. All the centers are tertiary university hospitals with immediate cardiac surgery facilities, and are leaders in treatment and research in IE.
The participating centers have ongoing prospective local databases including all consecutive patients with IE admitted to their institutions. A standardized case report form for each patient was recorded at each site. The protocols conformed to the ethics guidelines of the 1975 Declaration of Helsinki and its subsequent revisions and were approved by the local ethics committees. The proportion of missing data was <10% in all analyzed variables.
We included only patients with definitive LSIE according to the Duke criteria until 2002 and the modified Duke criteria thereafter.10,11
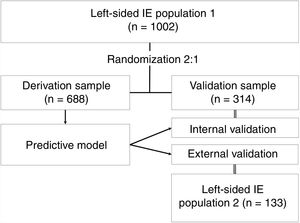
Study designThe predictive model was derived and internally validated using data from cohort 1 (n=1002). This population was randomized in a 2:1 proportion for the derivation and internal validation samples. Approximately two thirds of the population were used to derive the model (derivation sample, n=688) and the other third to validate it (internal validation sample, n=314). The predictive model was designed on the basis of the results of a multivariable analysis of in-hospital mortality including all the prognostic variables proposed by the European guidelines. The model was externally validated in cohort 2 (n=133). The study design is presented in figure 1.
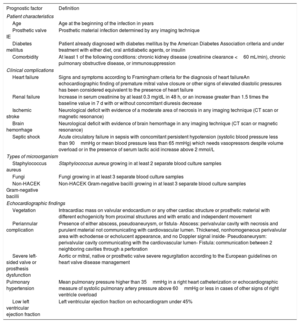
Definition of variablesA total of 17 out of the 19 prognostic variables proposed in the European Society of Cardiology (ESC) IE guidelines were included in our analysis. Variables were recorded during hospital admission, but only preoperatively in the case of cardiac surgery. Premature mitral valve closure and echocardiographic signs of elevated diastolic pressures were not included, as these factors were considered as surrogates of heart failure. In addition, the definition of some variables was adapted to achieve higher simplicity and reproducibility in the use of the predictive model. Severe left-sided valve regurgitation and severe prosthetic valve dysfunction were grouped together and valvular vegetation was considered irrespective of their length since there is no clear evidence-based cutoff point to consider a vegetation as large. The prespecified predictors and their definitions are summarized in table 1.
Definition of each prognostic factor used in the predictive model construction
| Prognostic factor | Definition |
|---|---|
| Patient characteristics | |
| Age | Age at the beginning of the infection in years |
| Prosthetic valve IE | Prosthetic material infection determined by any imaging technique |
| Diabetes mellitus | Patient already diagnosed with diabetes mellitus by the American Diabetes Association criteria and under treatment with either diet, oral antidiabetic agents, or insulin |
| Comorbidity | At least 1 of the following conditions: chronic kidney disease (creatinine clearance <60 mL/min), chronic pulmonary obstructive disease, or immunosuppression |
| Clinical complications | |
| Heart failure | Signs and symptoms according to Framingham criteria for the diagnosis of heart failureAn echocardiographic finding of premature mitral valve closure or other signs of elevated diastolic pressures has been considered equivalent to the presence of heart failure |
| Renal failure | Increase in serum creatinine by at least 0.3 mg/dL in 48 h, or an increase greater than 1.5 times the baseline value in 7 d with or without concomitant diuresis decrease |
| Ischemic stroke | Neurological deficit with evidence of a moderate area of necrosis in any imaging technique (CT scan or magnetic resonance) |
| Brain hemorrhage | Neurological deficit with evidence of brain hemorrhage in any imaging technique (CT scan or magnetic resonance) |
| Septic shock | Acute circulatory failure in sepsis with concomitant persistent hypotension (systolic blood pressure less than 90mmHg or mean blood pressure less than 65 mmHg) which needs vasopressors despite volume overload or in the presence of serum lactic acid increase above 2 mmol/L |
| Types of microorganism | |
| Staphylococcus aureus | Staphylococcus aureus growing in at least 2 separate blood culture samples |
| Fungi | Fungi growing in at least 3 separate blood culture samples |
| Non-HACEK Gram-negative bacilli | Non-HACEK Gram-negative bacilli growing in at least 3 separate blood culture samples |
| Echocardiographic findings | |
| Vegetation | Intracardiac mass on valvular endocardium or any other cardiac structure or prosthetic material with different echogenicity from proximal structures and with erratic and independent movement |
| Periannular complication | Presence of either abscess, pseudoaneurysm, or fistula- Abscess: perivalvular cavity with necrosis and purulent material not communicating with cardiovascular lumen. Thickened, nonhomogeneous perivalvular area with echodense or echolucent appearance, and no Doppler signal inside- Pseudoaneurysm: perivalvular cavity communicating with the cardiovascular lumen- Fistula: communication between 2 neighboring cavities through a perforation |
| Severe left-sided valve or prosthesis dysfunction | Aortic or mitral, native or prosthetic valve severe regurgitation according to the European guidelines on heart valve disease management |
| Pulmonary hypertension | Mean pulmonary pressure higher than 35mmHg in a right heart catheterization or echocardiographic measure of systolic pulmonary artery pressure above 60mmHg or less in cases of other signs of right ventricle overload |
| Low left ventricular ejection fraction | Left ventricular ejection fraction on echocardiogram under 45% |
CT, computed tomography; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium spp, Eikenella spp, Kingella spp; IE, infective endocarditis.
In-hospital mortality was used as the main event and included all-cause mortality during hospital stay. Antibiotic treatment and indications for surgery followed the recommendations of the European guidelines and decisions were taken by multidisciplinary experienced groups on IE. We considered urgent surgery to be surgery performed during the active phase of the disease, before the end of antibiotic treatment.12
Statistical analysisCategorical variables are reported as frequency (No.) and percentages and continuous variables as the mean±standard deviation or median and [interquartile range] in cases of nonnormal distribution. Normal distribution of quantitative variables was verified with the Kolmogorov-Smirnov test and visually through Q-Q plot graphics. Qualitative variables were compared with the chi-square test and Fisher exact test. Continuous variables were compared with the Student t test or its equivalent for nonparametric tests, the Mann-Whitney U test, for variables that were nonnormally distributed.
Randomization of cohort 1 was done by individual simple assignment of each episode with a probability of 0.67 for the derivation sample and a probability of 0.33 for the validation sample. We used the C4 Study Design Pack V 1.1 Glaxo Wellcome S.A. program.
Univariable analysis was performed in the derivation sample (cohort 1) to test the linear relation of each variable with the outcome, in-hospital mortality. To derive the predictive model, a logistic regression model with the maximum likelihood method using backward stepwise selection was adjusted, which included the prognostic factors shown in table 1. The ratio variable/event was controlled to avoid overfitting. For the final model, odds ratios (OR) adjusted for each of the variables included were calculated, along with their 95% confidence intervals (95%CI). This model was internally validated in the validation sample (cohort 1) and externally in cohort 2.
Noncollinearity was verified among the variables included in the model. The area under the receiver operating characteristic curve (ROC curve) was used to measure how well the model discriminated between patients with a high and low risk of in-hospital mortality. A value of 0.5 indicates no discrimination and a value equal to 1 indicates perfect discrimination. Calibration was evaluated with the Hosmer-Lemeshow test and with plots comparing predicted and observed mortality for different levels of risk.
P values are bilateral and were considered statistically significant with a P value <.05. Analyses were performed with the use of SPSS software, version 24.0 (IBM), and R software, version 3.4.3 (R Foundation for Statistical Computing).
RESULTSBaseline features of patients with left-sided infective endocarditisThe description of the main features in cohort 1 and the comparison between the derivation and internal validation samples resulting from its randomization are shown in . There were no relevant differences and the distribution of prognostic variables was homogeneous.
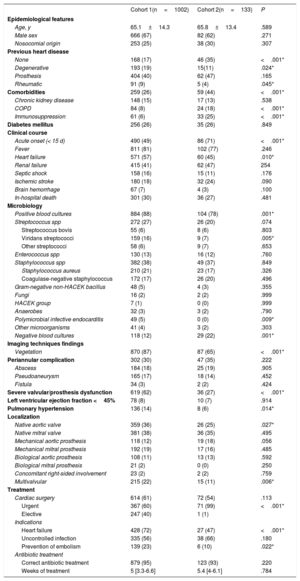
In addition, the main features of cohort 1 and cohort 2 were compared (table 2).
Comparison of populations in cohorts 1 and 2
| Cohort 1(n=1002) | Cohort 2(n=133) | P | |
|---|---|---|---|
| Epidemiological features | |||
| Age, y | 65.1±14.3 | 65.8±13.4 | .589 |
| Male sex | 666 (67) | 82 (62) | .271 |
| Nosocomial origin | 253 (25) | 38 (30) | .307 |
| Previous heart disease | |||
| None | 168 (17) | 46 (35) | <.001* |
| Degenerative | 193 (19) | 15(11) | .024* |
| Prosthesis | 404 (40) | 62 (47) | .165 |
| Rheumatic | 91 (9) | 5 (4) | .045* |
| Comorbidities | 259 (26) | 59 (44) | <.001* |
| Chronic kidney disease | 148 (15) | 17 (13) | .538 |
| COPD | 84 (8) | 24 (18) | <.001* |
| Immunosuppression | 61 (6) | 33 (25) | <.001* |
| Diabetes mellitus | 256 (26) | 35 (26) | .849 |
| Clinical course | |||
| Acute onset (< 15 d) | 490 (49) | 86 (71) | <.001* |
| Fever | 811 (81) | 102 (77) | .246 |
| Heart failure | 571 (57) | 60 (45) | .010* |
| Renal failure | 415 (41) | 62 (47) | 254 |
| Septic shock | 158 (16) | 15 (11) | .176 |
| Ischemic stroke | 180 (18) | 32 (24) | .090 |
| Brain hemorrhage | 67 (7) | 4 (3) | .100 |
| In-hospital death | 301 (30) | 36 (27) | .481 |
| Microbiology | |||
| Positive blood cultures | 884 (88) | 104 (78) | .001* |
| Streptococcus spp | 272 (27) | 26 (20) | .074 |
| Streptococcus bovis | 55 (6) | 8 (6) | .803 |
| Viridans streptococci | 159 (16) | 9 (7) | .005* |
| Other streptococci | 58 (6) | 9 (7) | .653 |
| Enterococcus spp | 130 (13) | 16 (12) | .760 |
| Staphylococcus spp | 382 (38) | 49 (37) | .849 |
| Staphylococcus aureus | 210 (21) | 23 (17) | .326 |
| Coagulase-negative staphylococcus | 172 (17) | 26 (20) | .496 |
| Gram-negative non-HACEK bacillus | 48 (5) | 4 (3) | .355 |
| Fungi | 16 (2) | 2 (2) | .999 |
| HACEK group | 7 (1) | 0 (0) | .999 |
| Anaerobes | 32 (3) | 3 (2) | .790 |
| Polymicrobial infective endocarditis | 49 (5) | 0 (0) | .009* |
| Other microorganisms | 41 (4) | 3 (2) | .303 |
| Negative blood cultures | 118 (12) | 29 (22) | .001* |
| Imaging techniques findings | |||
| Vegetation | 870 (87) | 87 (65) | <.001* |
| Periannular complication | 302 (30) | 47 (35) | .222 |
| Abscess | 184 (18) | 25 (19) | .905 |
| Pseudoaneurysm | 165 (17) | 18 (14) | .452 |
| Fistula | 34 (3) | 2 (2) | .424 |
| Severe valvular/prosthesis dysfunction | 619 (62) | 36 (27) | <.001* |
| Left ventricular ejection fraction <45% | 78 (8) | 10 (7) | .914 |
| Pulmonary hypertension | 136 (14) | 8 (6) | .014* |
| Localization | |||
| Native aortic valve | 359 (36) | 26 (25) | .027* |
| Native mitral valve | 381 (38) | 36 (35) | .495 |
| Mechanical aortic prosthesis | 118 (12) | 19 (18) | .056 |
| Mechanical mitral prosthesis | 192 (19) | 17 (16) | .485 |
| Biological aortic prosthesis | 108 (11) | 13 (13) | .592 |
| Biological mitral prosthesis | 21 (2) | 0 (0) | .250 |
| Concomitant right-sided involvement | 23 (2) | 2 (2) | .759 |
| Multivalvular | 215 (22) | 15 (11) | .006* |
| Treatment | |||
| Cardiac surgery | 614 (61) | 72 (54) | .113 |
| Urgent | 367 (60) | 71 (99) | <.001* |
| Elective | 247 (40) | 1 (1) | |
| Indications | |||
| Heart failure | 428 (72) | 27 (47) | <.001* |
| Uncontrolled infection | 335 (56) | 38 (66) | .180 |
| Prevention of embolism | 139 (23) | 6 (10) | .022* |
| Antibiotic treatment | |||
| Correct antibiotic treatment | 879 (95) | 123 (93) | .220 |
| Weeks of treatment | 5 [3.3-6.6] | 5.4 [4-6.1] | .784 |
CPOD, chronic obstructive pulmonary disease; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium spp, Eikenella spp, Kingella spp.
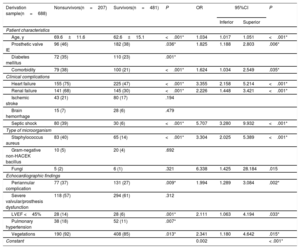
Table 3 shows the relationship between the variables proposed by the European guidelines and in-hospital mortality in the derivation sample (n=688). All variables, except ischemic stroke, cerebral hemorrhage, fungi, non-HACEK Gram-negative bacilli (Haemophilus spp, Aggregatibacter spp, Cardiobacterium spp, Eikenella spp, Kingella spp) and severe valve/prosthesis dysfunction, were statistically associated with in-hospital mortality in the univariable analysis.
Association between in-hospital mortality and variables proposed by the European guidelines on IE in cohort 1 (derivation sample)
| Derivation sample(n=688) | Nonsurvivors(n=207) | Survivors(n=481) | P | OR | 95%CI | P | |
|---|---|---|---|---|---|---|---|
| Inferior | Superior | ||||||
| Patient characteristics | |||||||
| Age, y | 69.6±11.6 | 62.6±15.1 | <.001* | 1.034 | 1.017 | 1.051 | <.001* |
| Prosthetic valve IE | 96 (46) | 182 (38) | .036* | 1.825 | 1.188 | 2.803 | .006* |
| Diabetes mellitus | 72 (35) | 110 (23) | .001* | ||||
| Comorbidity | 79 (38) | 100 (21) | <.001* | 1.624 | 1.034 | 2.549 | .035* |
| Clinical complications | |||||||
| Heart failure | 155 (75) | 225 (47) | <.001* | 3.355 | 2.158 | 5.214 | <.001* |
| Renal failure | 141 (68) | 145 (30) | <.001* | 2.226 | 1.448 | 3.421 | <.001* |
| Ischemic stroke | 43 (21) | 80 (17) | .194 | ||||
| Brain hemorrhage | 15 (7) | 28 (6) | .479 | ||||
| Septic shock | 80 (39) | 30 (6) | <.001* | 5.707 | 3.280 | 9.932 | <.001* |
| Type of microorganism | |||||||
| Staphylococcus aureus | 83 (40) | 65 (14) | <.001* | 3.304 | 2.025 | 5.389 | <.001* |
| Gram-negative non-HACEK bacillus | 10 (5) | 20 (4) | .692 | ||||
| Fungi | 5 (2) | 6 (1) | .321 | 6.338 | 1.425 | 28.184 | .015 |
| Echocardiographic findings | |||||||
| Periannular complication | 77 (37) | 131 (27) | .009* | 1.994 | 1.289 | 3.084 | .002* |
| Severe valvular/prosthesis dysfunction | 118 (57) | 294 (61) | .312 | ||||
| LVEF <45% | 28 (14) | 28 (6) | .001* | 2.111 | 1.063 | 4.194 | .033* |
| Pulmonary hypertension | 38 (18) | 52 (11) | .007* | ||||
| Vegetations | 190 (92) | 408 (85) | .013* | 2.341 | 1.180 | 4.642 | .015* |
| Constant | 0.002 | < .001* | |||||
95%CI, 95% confidence interval; IE, infective endocarditis; HACEK, Haemophilus spp, Aggregatibacter spp, Cardiobacterium spp, Eikenella spp, Kingella spp; LVEF, left ventricular ejection fraction; OR: odds ratio.
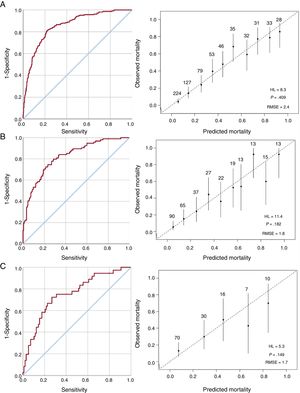
Then, a multivariate analysis was undertaken (table 3). Independent predictors of in-hospital mortality were age, prosthetic valve IE, comorbidities, heart failure, renal failure, septic shock, Staphylococcus aureus, fungi, periannular complications, ventricular dysfunction, and vegetations. The model showed good discriminatory ability with an area under the ROC curve of 0.855 (95%CI, 0.825-0.885) and good calibration (figure 2A).
Discriminatory performance and calibration of the model. A: ROC curve and plot comparing predicted and observed in-hospital mortality in the derivation sample. B: ROC curve and plot comparing predicted and observed in-hospital mortality in the internal validation sample. C: ROC curve and plot comparing predicted and observed in-hospital mortality in the external validation sample. HL, Hosmer-Lemeshow; RMSE, root mean square error; ROC, receiver operating characteristic.
The formula to predict in-hospital mortality was built by using the logarithms of adjusted OR from the predictive model:
Where z=– 6.288+0.033 x Age+0.602 x Prosthetic valve IE+0.485 x Comorbidity+1.210 x Heart failure+0.800 x Renal failure+1.742 x Septic shock+1.195 x Staphylococcus aureus+1.847 x Fungi+0.690 x Periannular complication+0.747 x Low left ventricular ejection fraction+0.850 x Vegetation.
Model validationThe model was internally and externally validated with the internal validation sample from cohort 1 (n=314) and from cohort 2 (n=133), respectively. Internal validation showed an area under the ROC curve of 0.823 (95%CI, 0.774-0.873). The model predicted an in-hospital mortality of 30.7% (95%CI, 27.7-33.7) and observed mortality was 29.9% (figure 2B).
External validation showed an area under the ROC curve of 0.753 (95%CI, 0.659-0.847). The model predicted an in-hospital mortality of 26.4% (95%CI, 22.2-30.5) and observed mortality was 29.9% (figure 2C).
Presentation of the modelThe model can be accessed as an informatic application via internet at ENDOVAL score web13 and via google play store (“ENDOVAL score”).
DISCUSSIONWe present the first predictive model of in-hospital mortality in LSIE derived by using the prognostic factors proposed by the European guidelines on the management of IE. Our results show that the model has high discriminatory ability.
Prognosis assessment in left-sided infective endocarditisDiagnosis and treatment of IE is a clinical challenge. Early identification of patients with LSIE at high risk is crucial to change the natural course of the disease.8 Previous important research has focused on the prognosis of IE.1,4,5,7,14–16 Although some of these classic studies on IE present a very good overview of the disease, they have important methodological limitations. First, these studies did not differentiate between left- and right-sided IE episodes, despite having very different profiles and prognosis.1,4,14 Furthermore, most studies focused on evaluating a single or a limited number of prognostic factors.3,11,12,17–31 The European guidelines summarize the most important prognostic factors in an attempt to reflect current knowledge and help clinicians in their daily practice; however, the information is not sufficiently accurate and its practical usefulness is limited. We tested the prognostic power of these prespecified variables, as we consider that all of them have clinical importance and have the scientific support of the authors and reviewers of the guidelines.
Practical implicationsOur group published a very simple prognostic stratification of patients with LSIE determined at admission and based on the presence of heart failure, Staphylococcus aureus, and periannular complications.15 Our new predictive model is a simple tool to help obtain a quick and accurate estimate of patient prognosis. This should not be regarded as definitive but as a complementary source of prognostic information that, together with other variables, will help clinicians decide whether and when surgery is indicated. It can be inferred from our results that in-hospital mortality risk can be assessed for the same patient at different time points in the course of the disease, but this hypothesis must be confirmed in prospective studies. In addition, the model also may help patients and families to obtain accurate information and a better understanding of the disease and its complications.
Differential features of our workOur work has some strengths. This study includes only patients with definite LSIE. The number of episodes is high in a disease that has a low incidence, and information from 4 tertiary hospitals has been included. The information is homogeneous and of high quality. Finally, the study focused on the prognostic factors proposed by the European guidelines, and demonstrates their prognostic power for the first time. This methodology precludes the bias that could exist in our population in the selection of variables for the construction of the predictive model, and favors the generalization of our results.
LimitationsThis work also has some limitations. All centers are tertiary hospitals with cardiac surgery facilities and are leaders in IE management, which restricts the applicability of the model to hospitals with similar characteristics. Cohort 1 included patients between 2000 and 2017, a long period during which different forms of management have been tested, which could have limited the accuracy of the model. The external validation cohort is more recent, which could explain some of the differences between cohorts and could be considered as a methodological shortcoming. Although the good performance of the model in the validation cohort reinforces the clinical usefulness of our work, future external validations, particularly with larger sample sizes and different case-mix populations, would improve the applicability of the predictive model. The definition of variables in the European guidelines is sometimes somewhat simple and, at other times, includes small adaptations that could have limited the prognostic impact of those variables.
Finally, the inclusion of other prognostic variables may improve the predictive performance of the model; however, for the sake of simplicity and general applicability, we tested only variables proposed by the European guidelines. Future investigations will be necessary to validate the results and to explore the effect of including new variables.
CONCLUSIONSOur predictive model of in-hospital mortality in left-sided IE based on the prognostic variables proposed by the ESC IE guidelines has high discriminatory ability.
FundingThis work was supported by Gerencia Regional de Salud de la Junta de Castilla y León [GRS 1523/A/17].
Conflicts of interestNone.
LSIE mortality is high and remains steady despite important medical advances. There are several known prognostic factors that are summarized by the European guidelines on IE in an attempt to reflect current knowledge and help clinicians in their daily practice; however, the information is not sufficiently accurate and its practical usefulness is limited.
WHAT DOES THIS STUDY ADD?This study adds a predictive model of in-hospital mortality in left-sided IE with high discriminatory ability, based on the prognostic variables proposed by the ESC IE guidelines. This model emerges as a tool to help in the decision-making process of the endocarditis team by giving a quick and accurate estimate about patient prognosis. In addition, the model may also help patients and families to obtain accurate information and a better understanding of the disease and its complications.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2019.11.003