The ADVANCE III trial showed that a delayed-detection strategy reduces implantable cardioverter-defibrillator (ICD) therapies. Here, we describe the adherence to and predictors of ADVANCE adoption and compare ICD therapy rates between patients with and without ADVANCE programming.
MethodsThis observational retrospective study analyzed patients implanted with Medtronic ICDs included from 2005 to 2016 in a Spanish national multicenter registry (UMBRELLA database; ClinicalTrials.gov, NCT01561144). Changes in ADVANCE programming adoption were described in relation to a) publication of the ADVANCE trial, b) implementation of an “ADVANCE awareness” campaign, and c) publication of an expert consensus statement. Multivariate logistic regression identified predictors of adoption. Therapy incidence rates were compared between groups by estimating the adjusted incidence rate ratio (aIRR) using negative binomial regression.
ResultsA total of 3528 patients were included. An ADVANCE strategy was used in 20% overall and in 44% at the end of the study. ADVANCE III adoption increased after trial publication, with less growth after an “ADVANCE awareness” campaign and after expert consensus statement publication. Predictors of ADVANCE adoption were as follows: ICD device with a nominal number of intervals to detect 30/40 (aOR, 4.4; 95%CI, 3.5-5.4), implantation by an electrophysiologist (aOR, 1.7; 95%CI, 1.4-2.2), and secondary prevention (aOR, 3.2; 95%CI, 2.6-3.9). Dual-chamber ICDs (aOR, 0.6; 95%CI, 0.5-0.8) and cardiac resynchronization-defibrillators (aOR, 0.5; 95%CI, 0.4-0.7) were associated with lower adoption. ADVANCE programming was associated with reduced total therapy burden (aIRR, 0.77; 95%CI, 0.69-0.86) and fewer inappropriate shocks (aIRR, 0.66; 95%CI, 0.52-0.85).
ConclusionsADVANCE adoption remains modest and can be improved through evidence-driven selection of nominal ICD settings. ADVANCE programming is associated with reduced therapy rates in real-world ICD recipients.
Keywords
Implantable cardioverter-defibrillators (ICDs) have become one of the cornerstones in the management of patients at risk of sudden arrhythmic death.1,2 Increased implantation of ICDs has drawn attention to their potential short- and long-term complications. Specifically, multiple studies have identified ICD shocks, whether appropriate or inappropriate, as a potential cause of worsening heart failure and mortality.3,4 This has led to the development of strategies aimed at reducing both inappropriate and potentially “unnecessary” ICD shocks. In this context, the ADVANCE III trial showed that the use of a delayed-detection strategy for arrhythmia detection reduced ICD therapies.5 We thus designed a study with 3 main objectives: a) to describe adherence to an “ADVANCE programming strategy” in real-world ICD recipients; b) to identify predictors of the adoption of such a strategy; and c) to compare ICD therapy rates in patients managed with ADVANCE vs standard ICD programming.
METHODSStudy design and populationThis is an observational, comparative, retrospective cohort analysis of a prospective registry. Baseline and follow-up data were extracted from the UMBRELLA database (ClinicalTrials.gov, NCT01561144). This multicenter registry included all patients implanted with a remote monitoring-capable Medtronic ICD (both new implants and generator replacements) in Spain since January 2005 (details of the registry have been published elsewhere6). Thirty-eight centers participated in the registry. All consecutive patients prospectively enrolled in the registry between 2005 and April 2016 and followed with the CareLink remote monitoring system were included in the analysis. Devices were programmed according to physician discretion. Patient follow-up included remote monitoring with the CareLink system as well as regular visits to ICD clinics according to the local protocol. Patients were followed up until their last remote transmission. All stored ICD events were analyzed by 2 of 3 expert electrophysiologists (who were blinded to individual patient programming) and classified as appropriate or inappropriate. In the case of disagreement, the episode was discussed in an event review committee meeting with all 3 experts.
For the purpose of the analysis, patients were divided into 2 groups according to device programming at implantation (specifically, at the time of discharge; programming changes during follow-up were not assessed): a) an ADVANCE group, which was defined as per the original trial (number of intervals to detect [NID] 30/40, ventricular fibrillation detection window <320ms, and antitachycardia pacing before or during charging), and b) a “non-ADVANCE” programming group (all patients not meeting ADVANCE programming criteria). Furthermore, primary prevention patients were required to have no additional “active” zones below the ventricular fibrillation window to be considered ADVANCE-programmed, whereas such zones were permitted in secondary prevention patients.
Changes in the percentage of patients who were programmed to ADVANCE therapy settings at implantation were described in relation to 3 events: publication of the ADVANCE trial results (May 2013), implementation of an “ADVANCE awareness” campaign for Medtronic technical consultants (January 2015), and publication of the HRS/EHRA/APHRS/SOLAECE ICD programming expert consensus statement on optimal ICD programming and testing (November 2015).7 Accordingly, 4 time periods were defined: period 1 (before publication of the ADVANCE III results), period 2 (from ADVANCE III publication to the “ADVANCE awareness” campaign), period 3 (from the “ADVANCE awareness” campaign to the expert consensus publication), and period 4 (after the expert consensus statement). Adoption rates were calculated for each trimester by dividing the number of implanted patients receiving ADVANCE programming by the total number of implantations performed during each 3-month period.
The “ADVANCE awareness” campaign comprised a series of lectures given between November 2014 and January 2015 by a senior electrophysiologist and attended on a compulsory basis by all Medtronic technical consultants, who in Spain assist in all ICD implantation/generator replacement procedures. The aim of the lectures (consisting of 2 separate 1-hour sessions) was to familiarize Medtronic technical consultants with the then-current evidence on device programming and specifically with the programming strategy used in the “active” arm of the ADVANCE III trial, with instructions to advise the implanting physician to adopt such a strategy. A “tip card” with the recommended settings was provided to all Medtronic technical consultants ( and ), and the presentation was made available to all attendees to encourage feedback and interaction with lecturers.
Statistical methodsCategorical variables are expressed as counts and percentages and continuous variables as mean± standard deviation. Categorical variables were compared using chi-square test or Fisher exact test as appropriate, whereas continuous variables were compared using t test, after assessment of normality with Kolmogorov-Smirnov tests. Left ventricular ejection fraction was categorized as <30%, 30%-49%, and ≥ 50%.
First, a segmented regression model was used to evaluate the contribution of each of the 3 above-mentioned events (trial results publication, awareness campaign, and expert consensus statement) to the adoption of an ADVANCE III programming strategy at implantation, with each of these events entered as a “breakpoint” in the model. For each time segment (period), the y intercept (representing the adoption rate at the beginning of each time period) and slope (“b”, representing the rate of increase in ADVANCE adherence during the period) were parameterized. Each point in the time series (representing a 3-month window) was weighted according to the total number of implantations performed during that particular 3-month interval. Goodness-of-fit was assessed with the R2, mean absolute error, and root-mean-square error statistics.
Second, multivariate logistic regression was used to identify independent predictors of ADVANCE III adoption. Variables showing an association with ADVANCE adoption in univariate analysis were included in the multivariate model (model entry criteria: univariate P <.2).
Third, device therapy burden in each group was described as ICD therapy incidence rates, which were expressed as the number of therapies per 100 patient-years. To compare ICD therapy incidence rates between patients with and without ADVANCE programming, adjusted incidence rate ratios (aIRRs) with 95% confidence intervals (95%CIs) were calculated using multivariate negative binomial regression, which was selected to account for overdispersion of the dependent variables (ie, ICD therapy rates) as defined by excess variance relative to mean. Overdispersion is primarily caused by a lack of independence between counted events (ie, the number of ICD therapies per patient), which arises when the occurrence of an event (device therapy) affects the probability of further events. Negative binomial regression includes a dispersion parameter in the model whereby estimated confidence intervals are adjusted for variability in the number of events per patient. Three separate multivariate models were constructed as follows for: a) any ICD therapy; b) appropriate ICD shock; and c) inappropriate ICD shock. Each of the 3 models was adjusted by the inclusion of variables that: a) could act as plausible confounders, and b) exhibited a P value <.20 in univariate analysis. Each model was offset to account for differences in the duration of follow-up between the ADVANCE and non-ADVANCE groups, using the natural log transformation of person-years to model exposure.
Statistical significance was set at a P value of .05. Statistical analysis was performed using SPSS version 17.
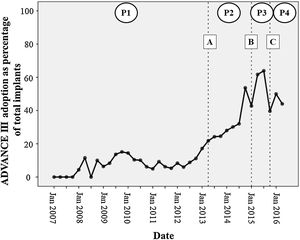
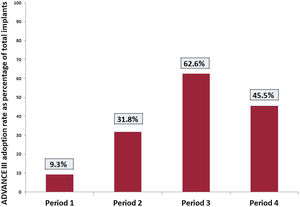
RESULTSReal-world adoption of an ADVANCE III programming strategyThe analysis included 3528 patients who received an implant between January 2005 and April 2016. Overall, an ADVANCE strategy was selected in 717 patients (20.3%) at device implantation. ADVANCE programming was first used in late 2007, which thus serves as the starting point in the historical analysis. ADVANCE penetration began at 8.7%, with almost no growth during period 1 (b=0.0005). The average adoption rate was 9.3% during this period. During period 2, ADVANCE penetration began at 20.8% and exhibited a 3.8% increase per trimester, leading to an average adherence of 31.8% during this period. ADVANCE programming penetration during period 3 increased by 2.2% per trimester; with an average adoption of 62.6% during the period. During period 4, after publication of the ICD programming expert consensus, adherence to an ADVANCE programming strategy started at 40% and increased by 2.5% per quarter, giving an average adoption during this period of 45.2%. ADVANCE penetration at the end of the study period was 44%. The changes over time in the adoption of an ADVANCE strategy at implantation are presented in figure 1, and the average adoption during each of the 4 periods is summarized in figure 2.
Temporal trends in the adoption (proportion of all implants) of an ADVANCE programming strategy at device implantation. A: publication of the ADVANCE III trial results. B: the “ADVANCE III awareness” training campaign for Medtronic technical consultants. C: publication of the 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement. P1, period 1; P2, period 2; P3, period 3; P4, period 4.
Average ADVANCE III adoption rate at implantation for each study period. Period 1: from 2007 (first ADVANCE-programmed patient in the registry) to publication of the ADVANCE III trial results (May 2013). Period 2: from publication of the ADVANCE III trial results to implementation of an “ADVANCE III awareness” training campaign for Medtronic technical consultants (January 2015). Period 3: from implementation of an “ADVANCE III awareness” training campaign for Medtronic technical consultants to release of the 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on ICD programming (November 2015). Period 4: after release of the HRS/EHRA/APHRS/SOLAECE expert consensus statement.
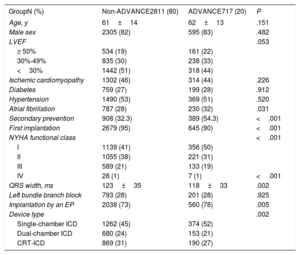
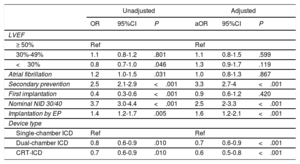
Baseline characteristics of patients in the ADVANCE and non-ADVANCE programming groups are shown in table 1. Univariate and multivariate predictors of ADVANCE adoption are presented in table 2. In univariate analysis, the following variables were associated with adherence to an ADVANCE programming strategy: history of atrial fibrillation, secondary prevention indication, a nominal (ie, “out-of-the-box” factory setting) NID 30/40, single-chamber ICD, and implantation by an electrophysiologist. Left ventricular ejection fraction <30% was negatively associated with ADVANCE programming (vs left ventricular ejection fraction> 50%).
Baseline characteristics of the study population
| GroupN (%) | Non-ADVANCE2811 (80) | ADVANCE717 (20) | P |
|---|---|---|---|
| Age, y | 61±14 | 62±13 | .151 |
| Male sex | 2305 (82) | 595 (83) | .482 |
| LVEF | .053 | ||
| ≥ 50% | 534 (19) | 161 (22) | |
| 30%-49% | 835 (30) | 238 (33) | |
| <30% | 1442 (51) | 318 (44) | |
| Ischemic cardiomyopathy | 1302 (46) | 314 (44) | .226 |
| Diabetes | 759 (27) | 199 (28) | .912 |
| Hypertension | 1490 (53) | 369 (51) | .520 |
| Atrial fibrillation | 787 (28) | 230 (32) | .031 |
| Secondary prevention | 908 (32.3) | 389 (54.3) | <.001 |
| First implantation | 2679 (95) | 645 (90) | <.001 |
| NYHA functional class | <.001 | ||
| I | 1139 (41) | 356 (50) | |
| II | 1055 (38) | 221 (31) | |
| III | 589 (21) | 133 (19) | |
| IV | 28 (1) | 7 (1) | <.001 |
| QRS width, ms | 123±35 | 118±33 | .002 |
| Left bundle branch block | 793 (28) | 201 (28) | .925 |
| Implantation by an EP | 2038 (73) | 560 (78) | .005 |
| Device type | .002 | ||
| Single-chamber ICD | 1262 (45) | 374 (52) | |
| Dual-chamber ICD | 680 (24) | 153 (21) | |
| CRT-ICD | 869 (31) | 190 (27) |
CRT-ICD, cardiac resynchronization therapy-implantable cardioverter-defibrillator; EP, electrophysiologist; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Data are expressed as No. (%) or mean±standard deviation
Univariate and multivariate predictors of adoption of an ADVANCE III programming strategy at device implantation
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | aOR | 95%CI | P | |
| LVEF | ||||||
| ≥ 50% | Ref | Ref | ||||
| 30%-49% | 1.1 | 0.8-1.2 | .801 | 1.1 | 0.8-1.5 | .599 |
| <30% | 0.8 | 0.7-1.0 | .046 | 1.3 | 0.9-1.7 | .119 |
| Atrial fibrillation | 1.2 | 1.0-1.5 | .031 | 1.0 | 0.8-1.3 | .867 |
| Secondary prevention | 2.5 | 2.1-2.9 | <.001 | 3.3 | 2.7-4 | <.001 |
| First implantation | 0.4 | 0.3-0.6 | <.001 | 0.9 | 0.6-1.2 | .420 |
| Nominal NID 30/40 | 3.7 | 3.0-4.4 | <.001 | 2.5 | 2-3.3 | <.001 |
| Implantation by EP | 1.4 | 1.2-1.7 | .005 | 1.6 | 1.2-2.1 | <.001 |
| Device type | ||||||
| Single-chamber ICD | Ref | Ref | ||||
| Dual-chamber ICD | 0.8 | 0.6-0.9 | .010 | 0.7 | 0.6-0.9 | <.001 |
| CRT-ICD | 0.7 | 0.6-0.9 | .010 | 0.6 | 0.5-0.8 | <.001 |
95%CI, 95% confidence interval; aOR, adjusted odds ratio; CRT-ICD, cardiac resynchronization therapy-implantable cardioverter-defibrillator; EP, electrophysiologist; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NID, number of intervals to detect; OR, odds ratio.
After multivariate analysis, a nominal NID 30/40 (adjusted odds ratio [aOR], 4.4; 95%CI, 3.5-5.4), secondary prevention indication (aOR, 3.2; 95%CI, 2.6-3.9), and implantation by an electrophysiologist (aOR, 1.7; 95%CI, 1.4-2.2) emerged as independent predictors of ADVANCE programming adoption, whereas patients implanted with either dual-chamber ICDs (aOR, 0.6; 95%CI, 0.5-0.8) or cardiac resynchronization therapy-ICDs (aOR, 0.5; 95%CI, 0.4-0.7) were less likely to be managed with an ADVANCE programming strategy.
Association between ICD programming and ventricular arrhythmia burdenThe average follow-up time was 49±26 months (52±26 months in the non-ADVANCE group and 39±22 months in the ADVANCE group). During the study period, 1265 patients (36% of the study population) experienced a total of 14 195 ICD therapies, resulting in an overall incidence rate of 102 therapies per 100 patient-years. In total, 215 ADVANCE patients (30%) experienced at least 1 device therapy vs 1050 (37%) in the non-ADVANCE group. The crude incidence rates are summarized in table 3, as well as the unadjusted and adjusted IRRs for each specific type of device therapy. The crude incidence rates of any appropriate therapy, appropriate shock, and inappropriate shock were 114.2, 16.6, and 5.2 therapies per 100 patient-years in the non-ADVANCE group vs 132.6, 21.9, and 4.1 therapies per 100 patient-years in the ADVANCE group.
Crude incidence rates and unadjusted and adjusted incidence rate ratios for each type of therapy
| Therapies per 100 patient-years | IRR | IRR (95%CI) | aIRR | aIRR (95%CI) | ||
|---|---|---|---|---|---|---|
| Non-ADVANCE | ADVANCE | |||||
| Any ICD therapy | 114.2 | 132.6 | 1.26 | 1.15-1.38 | 0.77 | 0.69-0.86 |
| ICD shock | 21.8 | 26.0 | 1.12 | 0.99-1.28 | 0.86 | 0.74-0.99 |
| ATP | 92.4 | 106.6 | 1.32 | 1.19-1.45 | 0.80 | 0.71-0.90 |
| Appropriate ICD therapy | 99.2 | 129.9 | 1.37 | 1.25-1.51 | 0.82 | 0.74-0.92 |
| Appropriate ICD shock | 16.6 | 21.9 | 1.29 | 1.14-1.48 | 1.02 | 0.88-1.17 |
| Appropriate ATP | 82.6 | 100.0 | 1.39 | 1.27-1.54 | 0.79 | 0.70-0.88 |
| Inappropriate ICD therapy | 15.0 | 10.7 | 0.67 | 0.57-0.78 | 0.52 | 0.43-0.62 |
| Inappropriate ICD shock | 5.2 | 4.1 | 0.74 | 0.59-0.94 | 0.66 | 0.52-0.85 |
| Inappropriate ATP | 9.8 | 6.6 | 0.66 | 0.54-0.8 | 0.54 | 0.44-0.68 |
95%CI, 95% confidence interval; aIRR, adjusted incidence rate ratio; ATP, antitachycardia pacing; ICD, implantable cardioverter-defibrillator; IRR, incidence rate ratio.
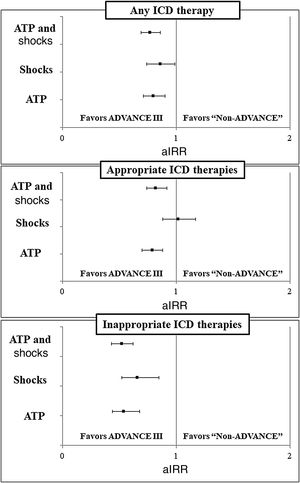
After multivariate adjustment, an ADVANCE programming strategy was associated with a reduction in any ICD therapy (aIRR, 0.77; 95%CI, 0.69-0.86), appropriate antitachycardia pacing (aIRR, 0.79; 95%CI, 0.7-0.88), inappropriate ICD shocks (aIRR, 0.66; 95%CI, 0.52-0.85), and inappropriate antitachycardia pacing (aIRR, 0.54; 95%CI, 0.44-0.68), with no significant differences in the incidence rates of appropriate ICD shocks (aIRR, 1.02; 95%CI, 0.88-1.17). The adjusted incidence rate ratios for each type of device therapy are presented as forest plots in figure 3. Univariate and multivariate predictors of each end point are provided in , and .
DISCUSSIONOur study shows that: a) real-world adoption of delayed-detection programming strategies for ICDs remains low, despite evidence of clinical benefit, b) strategies such as the publication of expert consensus statements or technical consultant “awareness” campaigns have little impact in the short--to-mid term; c) factors associated with adoption of delayed-detection programming are nominal ICD factory settings, implantation by an electrophysiologist, and secondary prevention indication; and d) strict adherence to a delayed-detection programming strategy is associated with reduced ICD therapy rates in real-world patients.
Clinical impact of adherence to an ADVANCE III strategyVarious trials have shown that prolonging arrhythmia detection times and restricting device therapy to very fast heart rates is associated with improved clinical outcomes,5,8,9 highlighting the concept of “avoidable” ICD therapies. The MADIT-RIT trial provided the first randomized evidence of improved outcomes with high-rate/delayed-detection programming in Boston Scientific ICDs, including reduced mortality.8 Shortly thereafter, the ADVANCE III trial showed similar results in Medtronic ICD recipients randomized to delayed therapy programming (NID 30/40 vs conventional programming with NID 18/24; with single-zone programming for primary prevention patients).5
In the present study, use of an ADVANCE programming strategy in daily practice was associated with a 23% reduction in any ICD therapy and a 34% reduction in inappropriate shocks. Appropriate shock rates were comparable in the 2 groups, as was also found in the MADIT-RIT, ADVANCE III, and PROVIDE trials. These data confirm, in an unselected real-world population of ICD recipients, the findings of the randomized ADVANCE III trial, which revealed a reduction in any device activation and in inappropriate shock burden. Our data also align with a recent study by Piccini et al., 10 who report the impact of contemporary programming strategies in a cohort of 64 769 patients, finding that high-rate and delayed-detection programming are associated with a reduced incidence of shocks. They also observed changes in programming trends over time, suggesting progressive penetration of the results of randomized trials into clinical practice.
Predictors of the adoption of a delayed-detection programming strategyOne of the major conclusions to be drawn from our study is that adoption of evidence-based ICD programming remains modest (< 50%) years after the publication of the pivotal randomized trials. Various studies have highlighted the gap between published scientific evidence and clinical practice.11–13 McGlynn et al.11 found that, among a random sample of 13 275 American patients, participants received only 54.9% of the recommended measures for basic care. On the other hand, although a time span of up to 17 years between the publication of randomized clinical trials and the implementation of findings into routine practice has been described,14 our results show an immediate increase in adoption rates after publication of the ADVANCE trial results. In fact, as shown in figure 1, adoption rates started increasing before publication of the study, likely due to the influence of previously published studies8,15,16 and a perception among physicians of the importance of avoidable therapies and the need for longer detection times. In this regard, it is important to note that period 1 encompasses a 6-year time span (2007-2013) and that the growth estimate that we provide is an average of that interval. Thus, overall, null growth characterized this period, despite the rise in ADVANCE programming observed just before trial publication.
With a study design similar to ours, Varma et al.17 analyzed the reaction to the publication of the MADIT-RIT trial and the 2015 consensus statement in a large US cohort using the ALTITUDE database (Boston Scientific). They described a baseline adherence to strict trial programming of <1% that increased to about 14% in the year following MADIT-RIT publication, with a <6% increase thereafter. Our results reveal higher penetration rates of the ADVANCE III programming schema. This increase might be related to the simpler design of the trial compared with the 3-arm MADIT-RIT study, which facilitated a more straightforward translation of the study findings to the 2015 consensus statement and to clinical practice. Moreover, a recent publication by Ananwattanasuk et al.18 also highlighted that, in a 3-center series of patients implanted with an ICD between 2014 and 2016, only one-third were programmed in accordance with the relevant guidelines.
Additionally, our results show that implanters are more likely to opt for ADVANCE III settings in single-chamber ICDs vs dual-chamber and cardiac resynchronization therapy-defibrillator devices. This could be attributed to the existence of fewer programmable features in single-chamber ICDs, which thus leads to “simpler” programming in these patients (notably, the MADIT-RIT trial did not include single-chamber ICDs). Additionally, reliance on dual-chamber discrimination criteria rather than prolonged detection could further explain the lower ADVANCE adherence among dual-chamber and cardiac resynchronization therapy-defibrillator devices.
A secondary prevention indication was likewise associated with increased adoption of ADVANCE programming. A plausible explanation for this finding is that, among the primary prevention patients included in our study, the enabling of “active” tachycardia zones below the ventricular fibrillation cutoff rate was one of the main reasons why these patients failed to meet a strict ADVANCE definition. On the other hand, the ADVANCE III trial allowed for additional ventricular tachycardia zones according to physician preference in secondary prevention individuals, making it easier for these patients to “meet” ADVANCE programming criteria in our study. Implantation by a certified electrophysiologist (vs a nonelectrophysiologist cardiologist or cardiac surgeon) was also associated with adherence to an ADVANCE approach.
Proposed strategies to improve adoption of evidence-based ICD programmingRegarding factors influencing programming choices, one of our most relevant findings is the strong association between nominal “out-of-the-box” settings (specifically, NID 30/40) and real-life programming practices. This finding highlights an opportunity for manufacturers to improve patient care by programming evidence-based default factory settings.
Further strategies that have been reported to improve ICD programming include center-specific feedback reports detailing adherence to evidence-based programming targets19 and active promotion of evidence, rather than passive reliance on the publication of scientific articles and guidelines.7,20 Our study shows that implementation of an “ADVANCE awareness” training campaign targeted at manufacturer technicians assisting physicians during implantations led to a continued increase in adoption rates, albeit at a “slower pace” vs the period after publication of the trial. Nevertheless, our results suggest a more prominent effect of trial publication on programming practices compared with technician training or the release of an expert consensus statement, a trend that was also observed in the study by Varma et al.17
Study limitationsAs with any retrospective design, unmeasured confounders influencing both the choice of programming and the association between device programming and therapy rates may have been overlooked by our study. For instance, no information was available on the number and type of antitachycardia pacing therapies programmed in each patient, which may influence shock rates. Our study concerns a single manufacturer; nevertheless, the accumulated evidence5,8,9 consistently shows the benefit of a delayed therapy strategy with all manufacturers evaluated thus far. In addition, we describe the programming practices at device implantation. Programming changes made at a later time were not captured in our dataset but may have influenced the subsequent therapy burden. Nonetheless, the finding by Varma et al.17 that <2% of patients in the ALTITUDE database who were re-programmed in any way during follow-up were re-programmed to MADIT-RIT settings suggests that substantial changes in programming during device follow-up are not the norm.
The time intervals between the Medtronic technical consultant campaign and the release of the expert consensus statement (“period 3”) and from then to the study closure date (“period 4”) were relatively short compared with the preceding time periods. This limited our ability to make definitive conclusions on the concrete effects of the campaign and the consensus statement. Nevertheless, our data point toward a clear picture: a marked increase in ADVANCE adoption following publication of the randomized trial results, which slowly tapers off despite the campaign and the expert consensus statement.
Finally, end-of-study vital status was lacking for a substantial number of patients (those who ceased transmissions through the remote monitoring platform may have done so due to device downgrade, generator replacement with that of a different manufacturer, or death), precluding a reliable analysis of mortality. Likewise, data on syncope rates were also unavailable.
CONCLUSIONSPenetration of an ADVANCE III programming strategy in clinical practice remains modest despite randomized evidence, an active promotion campaign, and expert consensus recommendations. ICD programming can be improved through evidence-driven selection of nominal “factory” settings. Adoption of an ADVANCE III programming strategy is associated with reduced therapy rates in real-world ICD recipients.
- -
ICDs have become one of the cornerstones in the management of patients at risk of sudden arrhythmic death.
- -
Multiple studies have identified ICD shocks, whether appropriate or inappropriate, as a potential cause of worsening heart failure and mortality.
- -
Several strategies have been explored in an attempt to reduce both inappropriate and potentially “unnecessary” ICD shocks.
- -
The ADVANCE III trial showed that the use of a delayed-detection strategy for arrhythmia detection reduced ICD therapies.
- -
Real-world adoption of delayed-detection programming strategies for ICDs remains low, despite evidence of clinical benefit.
- -
Strategies such as the publication of expert consensus statements or technical consultant “awareness” campaigns have little impact on day-to-day programming practices in the short-to-mid term.
- -
Adherence to guideline-recommended device parameters can be improved by evidence-driven selection of nominal “factory settings” by manufacturers, as well as ICD implantation by electrophysiologists.
- -
Adherence to a delayed-detection programming strategy is associated with reduced ICD therapy rates in real-world patients.
G. Loughlin has received funding from Medtronic for attendance at medical conferences. The original database used to conduct this study is the property of Medtronic. Medtronic did not take part in the study design, analysis, or preparation and writing of the manuscript. The remaining co-authors have no relevant conflicts of interests to declare.
The authors thank Miguel Ruiz for assistance with statistical analysis.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.06.017