Severe tricuspid regurgitation (TR) is a prevalent valve disease with a high mortality rate. Current guidelines do not define specific thresholds at which patients should be considered for surgery or percutaneous procedures. Thus, patients are usually referred for intervention at a late stage of the disease. This study aimed to assess predictors of cardiovascular outcomes in a prospective cohort of patients with severe TR referred for surgery.
MethodsThis was an observational, prospective, nonrandomized study. All patients underwent surgery for severe TR based on current clinical guidelines. Complete anamnesis, blood test, echocardiogram, cardiovascular magnetic resonance and right and left catheterization were performed. Patients were followed up in the outpatient department and a combined endpoint (hospitalization for heart failure and cardiovascular mortality) was registered.
ResultsForty-three consecutive patients were included (age: 66.9 ± 9.6 years, 67.4% female). Tricuspid annuloplasty was performed in all patients. After a median follow-up of 38 months, 12 patients (27.9%) showed the combined endpoint and 7 (16.3%) died. Above all clinical, blood and imaging data, the indexed right ventricular end-diastolic volume constituted the best predictor of the combined endpoint (HR, 1.1; P = .02) and cardiovascular mortality (HR, 1.1; P = .05). Furthermore, indexed right ventricular end-diastolic volume was associated with TR recurrence after surgery, with no impact on clinical outcomes.
ConclusionsIn patients with severe TR referred for surgery, right ventricular remodeling assessed by cardiovascular magnetic resonance constituted the best independent predictor of cardiovascular outcomes at follow-up.
Keywords
Severe tricuspid regurgitation (TR) is a common heart valve disease, affecting ≈1.6 million people in the United States and, if not treated, it results in progressive right ventricular (RV) dilation and failure, with an associated increase in morbidity and mortality.1 The prevalence of significant TR increases with age, nearing 1.5% and 5.6% in men and women > 70 years of age, respectively.2
Despite the increase in mortality associated with significant TR, management has classically been conservative.3 However, recent studies have shown chronic RV overload to be associated with irreversible RV dysfunction and poor prognosis. Thus, tricuspid valve repair at the time of left-sided valve surgery has recently gained acceptance.4,5 However, the optimal timing for surgery in isolated TR remains controversial and surgery is commonly undertaken at a late stage.3,4
Mortality in severe TR surgical series is high, ranging from 15% to 20% during hospitalization to 17% to 24% during the first year of follow-up.6 This high mortality rate has been attributed to delay in surgery since patients may develop anemia, RV dysfunction, cirrhosis, and/or renal failure.7 In a recent longitudinal echocardiographic study by Axtell et al.7 in a cohort of 3276 patients with severe TR, surgery was not associated with improved long-term survival compared with medical management alone after accounting for immortal time bias. Those authors hypothesized that a delay of up to 8 years from diagnosis to surgery could account for the absence of differences and recommended that the optimal timing for surgery be defined. If not, forthcoming randomized controlled trials with novel percutaneous techniques8,9 to address severe TR may fail to yield differences in mortality.
Data available to define the appropriate timing for surgery to improve survival in patients with severe TR are lacking. Thus, the present study aimed to define the main predictors of outcomes in a prospective cohort of patients with severe functional TR referred for surgery. The findings could be useful to plan the intervention (percutaneous or surgical) in this population to reduce outcomes at follow-up.
METHODSThis was an observational, single-center, prospective, nonrandomized study. The study was conducted in a public tertiary referral hospital with 1146 beds and more than 750 open-heart procedures per year. From April 2014 to December 2017, we included all consecutive patients with severe functional TR with an indication for surgery on the tricuspid valve (left-sided valve disease surgery could be associated if required). The indication for TR surgery was based on the current ESC guidelines for valvular heart disease management.10,11 Follow-up of patients with severe TR was based on annual 2-dimensional color Doppler echocardiography. Patients were referred to surgery when they had signs and symptoms of right heart failure refractory to optimal medical treatment as long as they did not present a RV ejection fraction < 30% or < 45% but associated with the presence of pulmonary arterial pressure ≥ 60mmHg (according to our study protocol).3 We excluded patients with a pacemaker, contraindication for cardiovascular magnetic resonance (CMR), congenital tricuspid valve disease, significant left-sided valvular heart disease (with an indication of surgical treatment per se), or severe pulmonary stenosis or regurgitation.
Patients included in the study were evaluated by anamnesis, complete physical examination, blood test, 2-dimensional color Doppler echocardiography, CMR, left cardiac catheterization to assess coronary artery disease and right cardiac catheterization to evaluate pulmonary pressure. Patients were followed up at the outpatient department and New York Heart Association (NYHA) functional class, hospitalization for heart failure and cardiovascular mortality were recorded. No functional tests were performed. The primary endpoint was the combination of hospitalization for heart failure and cardiovascular mortality at follow-up. The second endpoint was cardiovascular mortality alone. Cardiovascular operative mortality was included when it occurred within 30 days after surgery in or out of the hospital, and after 30 days during the same hospitalization subsequent to the intervention.12 Follow-up was started at the time of surgery.
Before discharge, a follow-up echocardiogram was performed in all patients who survived the first 24hours after surgery to determine the presence and the main predictors of residual TR.
Blood testBlood samples were collected in all patients and the following parameters were determined: hemoglobin, hematocrit, creatinine, estimated glomerular filtration rate, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, glutamyl transpeptidase, bilirubin, protein, albumin, renin, pro-brain natriuretic peptide, and aldosterone.
2-dimensional color Doppler echocardiographyAll patients were evaluated by echocardiography using Vivid 9 ultrasound (GE Healthcare, General Electric, USA) and all images were postprocessed with specific software: Echopach (GE Healthcare, General Electric, USA). The following parameters were evaluated: left and RV function, atrial and ventricular volumes and areas, tricuspid annulus diameter, tricuspid annular plane systolic excursion (TAPSE) and Doppler tissue imaging of the tricuspid ring (S’DTI). Valvular heart disease severity was assessed with the central jet area, vena contracta width, hepatic vein systolic flow, and density and shape of the continuous wave Doppler velocity profile as recommended by the current guidelines of the European Society of Cardiology.13 TR was classified according to the new proposed classification as mild, moderate, severe, massive, and torrential.14 In the case of a patient with associated mitral valve disease or mitral prosthesis, the study was completed by transesophageal echocardiography.
Cardiovascular magnetic resonanceAll CMR studies were performed with a 1.5 T clinical scanner (Sonata or Avanto scanner Siemens, Erlangen, Germany). Left ventricular ejection fraction (%), left ventricular end-diastolic volume index (mL/m2), LVESV index (mL/m2), left ventricular mass index (g/m2), RV ejection fraction (%), RV end-diastolic volume index (mL/m2), RV end-systolic volume index (mL/m2), global longitudinal, circumferential and radial strain of the free wall of the RV were calculated by CMR tissue tracking. In addition, right atrial area, diameters, and volumes were performed. Late gadolinium enhancement sequences were performed 10 to 15minutes after contrast administration. Further details on the technical aspects of CMR acquisition, sequences and quantification can be found in the .
CMR studies were analyzed off-line by an experienced observer blinded to all patient data using customized software (Cvi42, Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada).
Left and right cardiac catheterizationAll left and right cardiac catheterizations were performed using a Philips Integris Angio Diagnost 5 prior to surgery and according to current recommendations.15 The following parameters were evaluated: presence of significant coronary stenosis, systolic pulmonary artery pressure, mean pulmonary artery pressure, diastolic pulmonary artery pressure and indexed pulmonary vascular resistance. Results were analyzed by an experienced observer blinded to all patient data.
The study protocol was approved by the hospital Ethics Committee on human research and complied with the 1975 Declaration of Helsinki guidelines. All patients included in the study signed the consent form before inclusion.
Statistical analysisContinuous variables are expressed as mean ± standard deviation and categorical variables as the number of cases and proportions. Normal distribution was evaluated in continuous variables using the Shapiro-Wilk test and was compared among groups using the Student t test or Wilcoxon test. Categorical variables were compared using chi-square test or the Fisher exact test, as appropriate. Potential predictors of outcome were evaluated using proportional hazards Cox regression models. Variables were entered into the multivariate model if P-value < .10 on univariate analyses. To avoid multicollinearity, variables were excluded from the multivariable Cox regression if the tolerance test was < 0.1 or the variation inflation factor > 5. Schoenfeld residual analysis was also included to confirm that the proportional assumption was met for the exposure of interest. Martingale residuals distribution was performed to test the linearity assumption for the Cox models for the prediction of the composite endpoint and for the prediction of cardiovascular mortality based on the echocardiographic results before discharge. This analysis was an attempt to determine the main factors associated with a poor surgical outcome and not the time of its appearance. Receiver operator characteristics curves were used to define the discriminative ability of the logistical model to predict TR recurrence.
A P-value < .05 was considered statistically significant. SPSS 19.0 software version (IBM SPSS Statistics, Chicago, Illinois, USA) was used for the analysis.
RESULTSOver a 32-month period, 47 consecutive patients were enrolled in the study. However, 4 patients were excluded: 2 for severe claustrophobia and 2 for having a non-CMR conditional pacemaker. Thus, a final population of 43 patients was included in the analysis. Before surgery, all patients were under optimal medical treatment: beta-blockers in 11 patients (25.6%), digoxin in 26 (60.4%), angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in 34 (79%), mineralocorticoid receptor antagonist in 34 (79%) and diuretics in 43 (100%). Demographic characteristics are shown in table 1. Twenty-nine patients (67.4%) were women, mean age: 66.9 ± 9.6 years, 37 (86.1%) were in atrial fibrillation, and 97.6% were symptomatic (NYHA ≥ II). Nineteen patients (44.2%) had had prior surgery (57.9% mitral valve, 10.5% aortic valve, 10.5% mitral+aortic valves, 5.3% tricuspid valve, 5.3% mitral + tricuspid valve, 5.3% coronary artery bypass grafting, 5.3% others). TR was severe in 6 patients (13.9%) and massive in 37 (86%). None of the patients had torrential TR. Although the main indication for surgery was TR, 26 patients (60.5%) also required mitral valve repair (moderate secondary to endoleak), 4 (9.3%) aortic bioprosthesis for moderate aortic stenosis and 1 (2.3%) required tricuspid valve repair, mitral valve repair and aortic valve replacement (severe TR with moderate mitral regurgitation and moderate aortic stenosis). Tricuspid annuloplasty with a semirigid ring was performed in all patients. None of them received a valve prosthesis (bioprosthesis or a mechanical valve) since the study included only patients treated for secondary TR. Late gadolinium enhancement was not present in any of the patients.
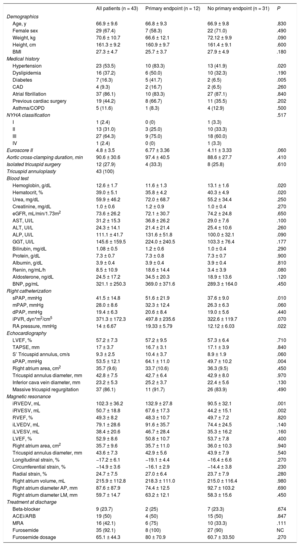
Baseline characteristics of the entire study group and of patients with or without the primary endpoint (hospitalization for heart failure or cardiovascular death)
| All patients (n = 43) | Primary endpoint (n = 12) | No primary endpoint (n = 31) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 66.9 ± 9.6 | 66.8 ± 9.3 | 66.9 ± 9.8 | .830 |
| Female sex | 29 (67.4) | 7 (58.3) | 22 (71.0) | .490 |
| Weight, kg | 70.6 ± 10.7 | 66.6 ± 12.1 | 72.12 ± 9.9 | .090 |
| Height, cm | 161.3 ± 9.2 | 160.9 ± 9.7 | 161.4 ± 9.1 | .600 |
| BMI | 27.3 ± 4.7 | 25.7 ± 3.7 | 27.9 ± 4.9 | .180 |
| Medical history | ||||
| Hypertension | 23 (53.5) | 10 (83.3) | 13 (41.9) | .020 |
| Dyslipidemia | 16 (37.2) | 6 (50.0) | 10 (32.3) | .190 |
| Diabetes | 7 (16.3) | 5 (41.7) | 2 (6.5) | .005 |
| CAD | 4 (9.3) | 2 (16.7) | 2 (6.5) | .260 |
| Atrial fibrillation | 37 (86.1) | 10 (83.3) | 27 (87.1) | .840 |
| Previous cardiac surgery | 19 (44.2) | 8 (66.7) | 11 (35.5) | .202 |
| Asthma/COPD | 5 (11.6) | 1 (8.3) | 4 (12.9) | .500 |
| NYHA classification | .517 | |||
| I | 1 (2.4) | 0 (0) | 1 (3.3) | |
| II | 13 (31.0) | 3 (25.0) | 10 (33.3) | |
| III | 27 (64.3) | 9 (75.0) | 18 (60.0) | |
| IV | 1 (2.4) | 0 (0) | 1 (3.3) | |
| Euroscore II | 4.8 ± 3.5 | 6.77 ± 3.36 | 4.11 ± 3.33 | .060 |
| Aortic cross-clamping duration, min | 90.6 ± 30.6 | 97.4 ± 40.5 | 88.6 ± 27.7 | .410 |
| Isolated tricuspid surgery | 12 (27.9) | 4 (33.3) | 8 (25.8) | .610 |
| Tricuspid annuloplasty | 43 (100) | |||
| Blood test | ||||
| Hemoglobin, g/dL | 12.6 ± 1.7 | 11.6 ± 1.3 | 13.1 ± 1.6 | .020 |
| Hematocrit, % | 39.0 ± 5.1 | 35.8 ± 4.2 | 40.3 ± 4.9 | .020 |
| Urea, mg/dL | 59.9 ± 46.2 | 72.0 ± 68.7 | 55.2 ± 34.4 | .250 |
| Creatinine, mg/dL | 1.0 ± 0.6 | 1.2 ± 0.9 | 1.0 ± 0.4 | .270 |
| eGFR, mL/min/1.73m2 | 73.6 ± 26.2 | 72.1 ± 30.7 | 74.2 ± 24.8 | .650 |
| AST, UI/L | 31.2 ± 15.3 | 36.8 ± 26.2 | 29.0 ± 7.6 | .100 |
| ALT, UI/L | 24.3 ± 14.1 | 21.4 ± 21.4 | 25.4 ± 10.6 | .260 |
| ALP, UI/L | 111.1 ± 41.7 | 131.6 ± 51.8 | 100.0 ± 32.1 | .090 |
| GGT, UI/L | 145.6 ± 159.5 | 224.0 ± 240.5 | 103.3 ± 76.4 | .177 |
| Bilirubin, mg/dL | 1.08 ± 0.5 | 1.2 ± 0.6 | 1.0 ± 0.4 | .290 |
| Protein, g/dL | 7.3 ± 0.7 | 7.3 ± 0.8 | 7.3 ± 0.7 | .900 |
| Albumin, g/dL | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.4 | .810 |
| Renin, ng/mL/h | 8.5 ± 10.9 | 18.6 ± 14.4 | 3.4 ± 3.9 | .080 |
| Aldosterone, ng/dL | 24.5 ± 17.2 | 34.5 ± 20.3 | 18.9 ± 13.6 | .120 |
| BNP, pg/mL | 321.1 ± 250.3 | 369.0 ± 371.6 | 289.3 ± 164.0 | .450 |
| Right catheterization | ||||
| sPAP, mmHg | 41.5 ± 14.8 | 51.6 ± 21.9 | 37.6 ± 9.0 | .010 |
| mPAP, mmHg | 28.0 ± 8.6 | 32.3 ± 12.4 | 26.3 ± 6.3 | .060 |
| dPAP, mmHg | 19.4 ± 6.3 | 20.6 ± 8.4 | 19.0 ± 5.6 | .440 |
| iPVR, dyn*m2/cm5 | 371.3 ± 172.3 | 497.8 ± 235.6 | 322.6 ± 119.7 | .070 |
| RA pressure, mmHg | 14 ± 6.67 | 19.33 ± 5.79 | 12.12 ± 6.03 | .022 |
| Echocardiography | ||||
| LVEF, % | 57.2 ± 7.3 | 57.2 ± 9.5 | 57.3 ± 6.4 | .710 |
| TAPSE, mm | 17 ± 3.7 | 16.7 ± 3.1 | 17.1 ± 3.9 | .840 |
| S’ Tricuspid annulus, cm/s | 9.3 ± 2.5 | 10.4 ± 3.7 | 8.9 ± 1.9 | .060 |
| sPAP, mmHg | 53.5 ± 12.1 | 64.1 ± 11.0 | 49.7 ± 10.2 | .004 |
| Right atrium area, cm2 | 35.7 (9.6) | 33.7 (10.6) | 36.3 (9.5) | .450 |
| Tricuspid annulus diameter, mm | 42.8 ± 7.5 | 42.7 ± 6.4 | 42.9 ± 8.0 | .970 |
| Inferior cava vein diameter, mm | 23.2 ± 5.3 | 25.2 ± 3.7 | 22.4 ± 5.6 | .130 |
| Massive tricuspid regurgitation | 37 (86.1) | 11 (91.7) | 26 (83.9) | .490 |
| Magnetic resonance | ||||
| iRVEDV, mL | 102.3 ± 36.2 | 132.9 ± 27.8 | 90.5 ± 32.1 | .001 |
| iRVESV, mL | 50.7 ± 18.8 | 67.6 ± 17.3 | 44.2 ± 15.1 | .002 |
| RVEF, % | 49.3 ± 8.2 | 48.3 ± 10.7 | 49.7 ± 7.2 | .820 |
| iLVEDV, mL | 79.1 ± 28.6 | 91.6 ± 35.7 | 74.4 ± 24.5 | .140 |
| iLVESV, mL | 38.4 ± 20.6 | 46.7 ± 28.4 | 35.3 ± 16.2 | .160 |
| LVEF, % | 52.9 ± 8.6 | 50.8 ± 10.7 | 53.7 ± 7.8 | .400 |
| Right atrium area, cm2 | 35.7 ± 9.6 | 35.7 ± 11.0 | 36.0 ± 10.3 | .940 |
| Tricuspid annulus diameter, mm | 43.6 ± 7.3 | 42.9 ± 5.6 | 43.9 ± 7.9 | .540 |
| Longitudinal strain, % | −17.2 ± 6.1 | −19.1 ± 4.4 | −16.4 ± 6.6 | .270 |
| Circumferential strain, % | −14.9 ± 3.6 | −16.1 ± 2.9 | −14.4 ± 3.8 | .230 |
| Radial strain, % | 24.7 ± 7.5 | 27.0 ± 6.4 | 23.7 ± 7.9 | .280 |
| Right atrium volume, mL | 215.9 ± 112.8 | 218.3 ± 111.0 | 215.0 ± 116.4 | .980 |
| Right atrium diameter AP, mm | 87.6 ± 87.9 | 74.4 ± 12.5 | 92.7 ± 103.2 | .690 |
| Right atrium diameter LM, mm | 59.7 ± 14.7 | 63.2 ± 12.1 | 58.3 ± 15.6 | .450 |
| Treatment at discharge | ||||
| Beta-blocker | 9 (23.7) | 2 (25) | 7 (23.3) | .674 |
| ACEi/ARB | 19 (50) | 4 (50) | 15 (50) | .847 |
| MRA | 16 (42.1) | 6 (75) | 10 (33.3) | .111 |
| Furosemide | 35 (92.1) | 8 (100) | 27 (90) | NC |
| Furosemide dosage | 65.1 ± 44.3 | 80 ± 70.9 | 60.7 ± 33.50 | .270 |
ACEi, angiotensin-conversing-enzyme inhibitors; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AP, anteroposterior; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; dPAP, diastolic pulmonary artery pressure; eGFR, glomerular filtration rate; GGT, gamma-glutamyltransferase; iLVEDV, indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; iPVR, indexed pulmonary vascular resistance; iRVEDV, indexed right ventricular end-diastolic volume; iRVESV, indexed right ventricular end-systolic volume; LM, lateral-medial; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; MRA, mineralcorticoid receptor antagonist; NC, not calculated; RA, right atrium; RVEF, right ventricular ejection fraction; sPAP, systolic pulmonary artery pressure.
Values are expressed as No. (%) or mean ± standard deviation.
All patients were followed up for a median of 38 months [interquartile range, 14-63 months].
Predictors of the primary endpointTwelve patients (27.9%) required hospitalization for heart failure or had a cardiovascular death. Median time from surgery to the event was 2.5 [0.3-15.3] months.
Compared with event-free patients, those with the primary outcome had a higher incidence of hypertension (P = .02), diabetes (P = .005), anemia (lower hemoglobin and hematocrit concentrations, P = .02 in both cases), higher indexed RV end-diastolic volume ([iRVEDV] 132.9mL/my vs 90.5mL/my, P = .001), higher indexed right ventricle end-systolic volume ([iRVESV] 67.6mL/my vs 44.2mL/my, P = .002), and higher systolic pulmonary arterial pressure estimated by echocardiography (64.1mmHg vs 49.7mmHg, P = .004) and right cardiac catheterization (P = .01) (table 1). The incidence of the primary event did not differ between patients who underwent isolated tricuspid surgery and those who associated a combined surgical approach (P = .61).
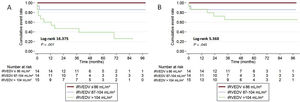
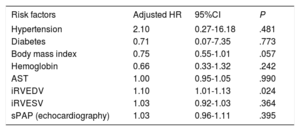
After adjustment for hypertension, diabetes, body mass index, hemoglobin, aspartate aminotransferase, iRVEDV, iRVESV, and systolic pulmonary arterial pressure variables, on multivariate Cox regression analysis, only iRVEDV was the main predictor of the primary outcome with a hazard ratio (HR) of 1.1 (95% confidence interval [95%CI], 1.01-1.13); P = .024 (table 2). The proportional hazards assumption was confirmed by Schoenfeld residual analysis (P = .90). The ability of iRVEDV to predict the primary endpoint was also confirmed by the C-statistic = 0.91 (95%CI, 0.74-0.95). Kaplan-Meier survival curves for the primary endpoint by iRVEDV tertiles are represented in figure 1A. In particular, the incidence of the primary outcome in patients with an iRVEDV > 104mL/m2 was 66% higher than in those with a lower threshold (10 patients vs 2, respectively; log-rank 16.375, P < .001).
Predictors of the primary endpoint (cardiovascular mortality and hospitalization for heart failure)
| Risk factors | Adjusted HR | 95%CI | P |
|---|---|---|---|
| Hypertension | 2.10 | 0.27-16.18 | .481 |
| Diabetes | 0.71 | 0.07-7.35 | .773 |
| Body mass index | 0.75 | 0.55-1.01 | .057 |
| Hemoglobin | 0.66 | 0.33-1.32 | .242 |
| AST | 1.00 | 0.95-1.05 | .990 |
| iRVEDV | 1.10 | 1.01-1.13 | .024 |
| iRVESV | 1.03 | 0.92-1.03 | .364 |
| sPAP (echocardiography) | 1.03 | 0.96-1.11 | .395 |
95%CI, 95% confidence interval; AST, aspartate aminotransferase; HR, hazard ratio; iRVEDV, indexed right ventricular end-diastolic volume; iRVESV, indexed right ventricular end-systolic volume; sPAP, systolic pulmonary artery pressure.
Cardiovascular operative mortality was 9.3% and overall mortality was 16.3% (all patients due to cardiovascular mortality). Median time from surgery to death was 2 [0.1-16] months. Four of the patients died before discharge (2 due to cardiogenic shock and 2 to acute RV dysfunction), and 3 died during follow-up (all due to RV heart failure).
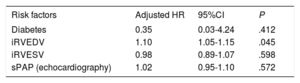
On univariate analysis, a rise in alkaline phosphatase (P = .014), a drop in albumin concentration (P = .031) and an increase in iRVEDV (P = .049) were associated with overall cardiovascular mortality (). However, on multivariate Cox regression analysis, iRVEDV was the main predictor of overall cardiovascular mortality with an HR of 1.1 (95%CI, 1.05-1.15); P = .045 (table 3). The proportion assumption was also confirmed by Schoenfeld residual analysis (P = .83). C-statistics also confirmed the high ability of iRVEDV to predict the secondary endpoint: 0.82 (95%CI, 0.65-0.92). Kaplan-Meier survival curves for cardiovascular mortality by iRVEDV tertiles are represented in figure 1B. Thus, patients with an iRVEDV > 104mL/my showed higher cardiovascular mortality than those with a lower iRVEDV (5 vs 2, respectively, log-rank 5.368; P = .045).
Predictors of cardiovascular death
| Risk factors | Adjusted HR | 95%CI | P |
|---|---|---|---|
| Diabetes | 0.35 | 0.03-4.24 | .412 |
| iRVEDV | 1.10 | 1.05-1.15 | .045 |
| iRVESV | 0.98 | 0.89-1.07 | .598 |
| sPAP (echocardiography) | 1.02 | 0.95-1.10 | .572 |
95%CI, 95% confidence interval; HR, hazard ratio; iRVEDV, indexed right ventricular end-diastolic volume; iRVESV, indexed right ventricular end-systolic volume; sPAP, systolic pulmonary artery pressure.
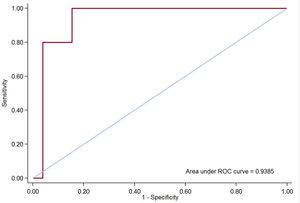
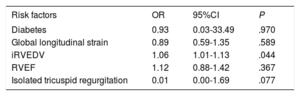
All patients (except 3 with early death) underwent a follow-up echocardiogram predischarge and TR recurrence was present in 32 patients: 19 (47.5%) mild, 8 (20.0%) moderate and 5 (12.5%) severe. Predictors of significant TR (degrees of regurgitation II or III/IV assessed by quantitative Doppler measures) recurrence are shown in . There was a rise in renin levels (P = .033), increased iRVEDV (P = .025) and an increase in RV function: RV ejection fraction (P = .009), RV free wall global longitudinal strain (P = .002), RV circumferential strain (P = .009) and RV radial strain (P = .025) were the main variables associated with significant TR recurrence, thereby suggesting that the better the RV function, the higher the velocity through the tricuspid valve, resulting in a higher Doppler signal of recurrent TR. However, on multivariate logistic regression analysis, only iRVEDV was associated with TR recurrence (OR, 1.06; 95%CI, 1.01-1.13; P = .044) (table 4). The area under the receiver operator characteristics curve (AUC) of the logistic model to predict TR recurrence was 0.939 (P < .01) (figure 2). Paradoxically, tricuspid annulus diameter presurgery was not associated with TR recurrence.
Multivariate analysis for significant tricuspid regurgitation recurrence
| Risk factors | OR | 95%CI | P |
|---|---|---|---|
| Diabetes | 0.93 | 0.03-33.49 | .970 |
| Global longitudinal strain | 0.89 | 0.59-1.35 | .589 |
| iRVEDV | 1.06 | 1.01-1.13 | .044 |
| RVEF | 1.12 | 0.88-1.42 | .367 |
| Isolated tricuspid regurgitation | 0.01 | 0.00-1.69 | .077 |
95%CI, 95% confidence interval; iRVEDV, indexed right ventricular end-diastolic volume; OR, odds ratio; RVEF, right ventricular ejection fraction.
Interestingly, the presence of recurrent significant TR (moderate or severe) was not associated with either the primary endpoint (HR, 2.26; 95%CI, 0.65-7.83; P = .197) or cardiovascular mortality (HR, 2.87; 95%CI, 0.48-17.21; P = .249).
DISCUSSIONTR has long been considered a forgotten entity owing to the belief that left-sided valve treatment prevented the development of significant TR.3 However, follow-up studies found this conservative approach to be associated with a high incidence of significant residual TR in 25% of patients.16,17 Thus, current ESC/EACTS11 and AHA/ACC18 guidelines for the management of valvular heart diseases recommend surgery in patients with mild or moderate secondary TR with a dilated annulus (≥ 40mm or > 21mm/m2 by 2-dimensional echocardiography) undergoing left-sided valve surgery (IIa and IIb class indication, respectively).
Significant TR is associated with increased morbidity and mortality.2,16 High functional class, RV dilation and dysfunction, pulmonary hypertension, renal failure, and severe liver dysfunction have been considered the main risk factors. The problem in patients with TR is that they can remain asymptomatic for a long time and, when symptoms appear, could be at an irreversible stage in which RV dysfunction persists despite treatment.6,7 A recent study by Axtell et al.7 in 3276 patients with isolated TR showed that surgical treatment performed up to 8 years after diagnosis was not associated with improved long-term survival compared with medical management alone and after adjustment for immortal time bias. Therefore, it is important to establish the precise moment when the surgical indication should be established to avoid progression to an irreversible stage. In addition, the new percutaneous approach for significant TR before irreversible RV dysfunction is established would reduce the mortality rate associated with surgical treatment. The need to recognize the ‘optimal timing’ for percutaneous treatment of the TR is vital to avoid unnecessary procedures.19,20 Our study is the first prospective work in patients with severe TR to evaluate the prognostic role of clinical, blood, echocardiographic, CMR and hemodynamic variables as predictors of major cardiac events (such as cardiovascular mortality and/or admission for heart failure). The main finding of our study was that RVEDVi assessed by CMR constituted the best predictor of cardiovascular outcomes during follow-up. Thus, RVEDVi > 104mL/m2 was associated with a 23.3% primary endpoint rate and an 11.6% cardiovascular mortality rate, with this value being independent of the degree of pulmonary hypertension or RV dysfunction severity. Kim et al.21 found that a RV end-systolic area < 20cm2 predicted event-free survival. Similarly, our results showed that a surgical intervention before significant RV dilation is present ( > 104mL/m2) could have reduced the primary endpoint incidence from 23.3% to 4.6% and overall mortality from 11.6% to 4.6%. In addition, our study is in line with a recent retrospective echocardiographic study by Dietz et al.22 who observed that the presence of RV dilation (defined as a tricuspid annulus ≥ 40mm) associated with ventricular dysfunction (defined as a TAPSE < 17mm) constituted the main predictors of global mortality in patients with severe TR. However, in that study, only 21% of the patients had a severe TR and only 8% underwent surgery. Similarly to our study, Kim et al.23 reported that, in 31 patients undergoing tricuspid valve surgery (especially tricuspid valve replacement in 81% of cases), an indexed end-diastolic volume of the RV < 164mL/m2 was the best predictor of recovery of RV function during follow-up. However, although patients in that study had a similar degree of RV dysfunction compared with that in our study (49.7 ± 8.3% vs 49.3 ± 8.2%), the threshold of iRVEDV obtained was clearly different (164mL/m2 vs 104mL/m2, respectively). Several reasons could explain these differences: the study by Kim et al. aimed to determine only RV function recovery without prognostic implications, the population was younger (56.6 ± 9.1 vs 66.9 ± 9.6 years, respectively), the proportion of male sex was lower (13% vs 33%, respectively), most patients underwent tricuspid valve replacement (81% vs 0%, respectively), systolic pulmonary arterial pressure value was not included and, finally, 6 patients who died at follow-up were excluded.23
Surgery is the treatment of choice for severe TR when medical treatment fails. However, it carries a high mortality rate (17%-24%)24 secondary to a late indication when patients have multiple comorbidities and is associated with irreversible RV remodeling. However, RV remodeling is not only influenced by TR severity, other factors such as pressure overload, cell death or myocardial fibrosis are also involved in the pathophysiology of the RV.22 Furthermore, similarto what occurs in the presence of severe mitral regurgitation, RV ejection fraction is not the best method for calculating the ventricular function in severe TR.25 Thus, in line with our results, the combination of RV dilatation/dysfunction associated with significant TR seems to be needed for right heart failure to occur,26,27 which is associated with tachyarrhythmias, ascites, liver dysfunction, lower cardiac output, and worse cardiovascular outcomes.26 The European11 and American18 guidelines for the management of heart valve diseases recommend surgery with indications IIa and IIb (respectively) in patients with severe TR when they are symptomatic or when they show progressive RV dilation/dysfunction, in the absence of severe left ventricular/RV dysfunction or significant pulmonary hypertension. However, the specific cutoff point at which surgery should be indicated has not been established, and the indication is thus made arbitrarily according to different centers. The overall cardiovascular mortality rate in our study was 16.3%. Moreover, the cardiovascular operative mortality rate was 9.3% similar to that reported by Verdonk et al.28 (5-13%) and lower than that reported by Rodríguez-Capitán et al.29 (18.5%). Unexpectedly, the combination of left valve surgery (required in 60.5% of patients with no severe left valve disease) was not associated with an increase in mortality. This could be explained by the fact that the indication for surgery in our population was based on the presence of TR severity and not on severe left valve disease. Furthermore, since all patients received optimal medical treatment at discharge (table 1) according to current clinical practice guidelines, inadequate treatment could not explain long-term mortality.
Similar to previous studies, in our series, most patients (97.6%) were symptomatic with a functional class (NYHA) ≥ II; however, compared with patients with no clinical outcomes, NYHA functional class was not statistically significant (P = .51) to predict outcomes, as published elsewhere.30,31 Moreover, various risk factors have been evaluated to stratify the risk of cardiovascular events in patients with significant TR. The presence of liver enzyme alterations, cirrhosis, or high bilirubin levels has been associated with increased mortality in tricuspid valve surgery.6,32 In addition, the presence of anemia has been associated with poor clinical outcomes.21 Several mechanisms have been associated with anemia in patients with significant TR such as low renal perfusion, malabsorption, nutritional deficiencies, hemodilution, and hypersplenism (secondary to systemic venous congestion).21 In our study, neither hemoglobin nor liver enzymes had prognostic implications. This could be explained by the fact that we evaluated a surgical population and therefore patients were not in the end-stage of the disease. The role of pulmonary arterial hypertension in patients undergoing TV surgery is controversial since some studies have shown that patients with pulmonary arterial hypertension do not recover RV function, while other studies have reported opposite findings.3,18 Our study demonstrated that pulmonary arterial hypertension is associated with a higher rate of cardiovascular events during follow-up with an HR of 1.05 (P = .004); however, its role in the prediction of outcomes is subject to the degree of RV dilation (P = .395, in the adjusted model).
LimitationsThe main limitations of our study are that it was a single-center study and with a small population; however, it does reflect the reality of this disease with few patients being referred for surgery (around 8% in big series)22 and mostly at an advanced stage of disease. Nevertheless, our study also has several strengths such as its prospective nature and the fact that all patients were studied within a homogeneous and exhaustive protocol that included all clinical data and imaging techniques recommended by scientific societies. A further limitation of our study is that patients were referred for surgery at different stages of their disease and no functional test rather than NYHA functional class was performed; however, current guidelines do not recommend specific thresholds for the performance of these tests. In this regard, our study provides an approximation of the indexed RV end-diastolic volume from which the risk of major cardiovascular complications after surgery is significantly increased during follow-up. Multivariate analysis can bias the results of the study when using a small sample size and, also, when the number of events is low. However, being an observational and prospective study, it minimizes the possibility of selection bias. This fact associated with the small differences between the groups with/without events allowed us to use smaller samples to obtain a good level of accuracy. Finally, recurrent TR regurgitation was analyzed based on quantitative Doppler analysis (since 3-dimensional transthoracic echocardiography was not performed in all patients sistematically).
CONCLUSIONsIn patients with isolated severe TR referred for surgical tricuspid valve repair, an end-diastolic RV volume > 104mL/m2 was associated with the need for hospitalization for RV heart failure and a high cardiovascular mortality rate during follow-up. These findings suggest that surgical valvular repair or percutaneous procedures should be performed before this threshold is reached in order to optimize results and improve patients’ quality of life.
FUNDINGThis work was partially funded by a Clinical Research Project Grant in Cardiology from the Spanish Society of Cardiology and Spanish Heart Foundation. There are no relationships with industry.
CONFLICTS OF INTERESTI. Ferreira-González is Editor-in-Chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors confirm they have no disclosures regarding this manuscript.
- -
Mortality in severe tricuspid regurgitation surgery is high (15%-20%) and is usually attributed to a delay in surgery. Despite this high mortality, there are no available data to define the best cardiovascular predictor at which patients should be referred for surgery to improve survival.
- -
This study shows that RV remodelling assessed by cardiac magnetic resonance is the best independent predictor of cardiovascular outcomes at follow-up in this population. In addition, survival could be reduced if patients are referred for surgery before an end-diastolic RV volume of 104 mL/m2 is reached.
We would like to thank Christine O’Hara for English revision.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.008