Malignancies are the second cause of death in developed countries after cardiovascular disease and both share common risk factors.
MethodsThis prospective study assessed the prevalence and postdischarge incidence of malignancies in all consecutive patients admitted for an acute coronary syndrome.
ResultsA total of 1819 patients were included. On admission, the prevalence of malignancies was 3.4%, and 41.9% of the patients were considered disease-free; of the 1731 discharged patients, the incidence was 3.1% (53 cases) and the most common locations were the colon, lung, bladder, and pancreas. Patients with prevalent malignancies were older and had more comorbidities and complications. There were no differences in the revascularization rate, but implantation of drug-eluting stents was less frequent in patients with prevalent malignancies. During follow-up, the median time to diagnosis of incident malignancies was 25 months. On multivariate analysis, independent risk factors were age and current or former smoking. All-cause mortality was much higher in patients with incident (64.2%) or prevalent (40.0%) malignancies. Multivariate analysis showed that prevalent and incident malignancies increased the risk of all-cause mortality by 4-fold.
ConclusionsAmong patients admitted for an acute coronary syndrome, 3.8% had a history of malignancy, with less than 50% considered cured. The incidence of new malignancies was 3.4% and both types of malignancies substantially impaired the long-term prognosis.
Keywords
Abbreviation
After cardiovascular disease, cancer is the second cause of death worldwide.1,2 Contrasting with the trend toward lower cardiovascular disease rates, the incidence of cancer has increased in recent decades, and cancer mortality has shown significant reductions only in the last 10 years.3 In 2012 alone, there were 3.5 million new cancer diagnoses in Europe and almost 2 million cancer deaths.4 Current data from the Spanish Cancer Registry Network (REDECAN in its Spanish initials) show that the most prevalent cancers in men are prostate, colon, lung, and bladder cancers, whereas the most common in women are breast, colon, uterine, and lung cancers.5 These rankings coincide with large European and American studies.3
Cardiovascular disease and cancer share several risk factors and it is therefore common for a patient to have both diseases.6 In recent years, interest has grown in the incidence and mortality of cardiovascular disease in cancer patients, revealing a cardiovascular cause in up to 30% of cancer-patient deaths.7,8 In contrast, in Spain, little attention has been paid to the prevalence and prognostic value of cancer in patients with acute coronary syndrome (ACS),9–11 and likewise there have been no reports on the incidence of de novo cancer in ACS patients. The aims of this study were first to describe the prevalence and type of cancer in patients admitted with ACS and then to analyze the incidence of de novo tumors during follow-up.
METHODSStudy DesignThis single-center prospective study included all 1819 consecutive patients admitted for ACS over a 7-year period. Patients with established cancer at the time of admission were identified from clinical histories, and records were kept of the diagnosis date, tumor location and type, specific treatments received, and whether the cancer was considered cured. Incident cancer was defined as cancer diagnosed after hospital discharge, and records were kept of the diagnosis date and the tumor location and type. During hospitalization, records were compiled of major diagnoses, disease histories, cardiovascular risk factors, treatments received, complementary investigations, and in-hospital thrombosis or bleeding. Histories of diabetes mellitus, dyslipidemia, and hypertension were recorded for patients receiving specific treatment or reporting a previous diagnosis of these diseases. Similarly, a history of obstructive lung disease was recorded for patients with a previous diagnosis or receiving specific medication. The glomerular filtration rate was determined from the serum creatine concentration using the Modification of Diet in Renal Disease Study equation.12 Comorbid conditions were analyzed using an adaptation of the Charlson comorbidity index for ischemic heart disease patients.13
Patients were followed up by review of clinical histories (including computerized patient records from primary care or emergency room consultations) and telephone interview. The primary outcome measure during follow-up was cardiovascular mortality and the secondary outcome was all-cause mortality.
Statistical AnalysisStatistical analysis was performed with STATA 14.2 and the R statistical package. Qualitative variables were evaluated with the chi-square test and the Fisher test when appropriate. Quantitative variables were compared by the Student t test and ANOVA (analysis of variance). After confirmation of the collinearity of age with the Charlson comorbidity index and the GRACE score, age was categorized until collinearity was no longer observed (> 75 years).
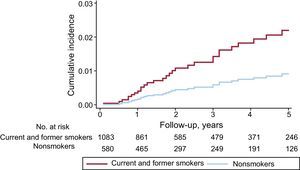
The outcomes analyzed during follow-up were first postdischarge cancer incidence and then prognosis in relation to whether patients developed cancer after discharge (incident cancer) or already had cancer on admission for ACS (prevalent cancer). For prognosis, we analyzed all-cause mortality, mortality due to cardiovascular causes, and noncardiovscular mortality. The detection of incident cancer could be affected by patient deaths during follow-up, potentially skewing analysis of the relationship between incidence and predictors. We therefore performed a competing risk analysis according to the Fine and Gray model.14 Variables were selected using the inclusion method; the included variables were age, sex, all risk factors, a history of heart failure, ischemic heart disease, atrial fibrillation or stroke, treatment at discharge, and coronary revascularization. Cumulative cancer incidence was plotted for current and former smoking, and differences were tested using the Gray method. The results of the multivariate analysis are presented as subdistribution hazard ratios (sHR) with corresponding 95% confidence intervals (95%CIs). The proportionality assumption was verified with the Schoenfeld residuals method, and calibration was measured using the C statistic.
Cancer incidence was an intermediate outcome between the start of follow-up and death as the final event, and the analysis therefore required the use of Markov multistate models. We defined 3 states (nonterminal longitudinal outcomes): a) no cancer; b) prevalent cancer; c) incident cancer. For the analysis of all-cause mortality, this event was added as a fourth state (the absorbing state); for the analysis of cardiovascular and noncardiovascular mortality, these events were added within the same model as states 4 and 5 (absorbing states). In this way, the results for different mortality causes were mutually adjusted for the effect of the competing risks that each had on the other. The results obtained correspond to the instantaneous hazard ratios for the transition between states, which are equivalent to the HR of traditional survival analysis. The analysis was performed with the “msm” package.15 Differences were considered statistically significant at P<.05.
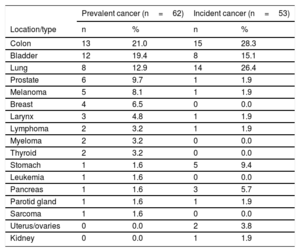
RESULTSCancer Prevalence on AdmissionThe prevalence of cancer on admission for ACS was 3.4% (95%CI, 2.7%-4.4%), and the most frequent tumor locations were colon, bladder, lung, and prostate (Table 1). The mean interval between cancer diagnosis and ACS was 5.5 years (interquartile range, 2.0-10.00 years). Of the patients with prevalent cancer, 74.2% had undergone specific cancer surgery, 46.8% chemotherapy, and 12.9% radiotherapy. The percentage of cancer patients considered disease-free on admission for ACS was 41.9%.
Cancer Present on Admission for Acute Coronary Syndrome and Developing During Follow-up
| Prevalent cancer (n=62) | Incident cancer (n=53) | |||
|---|---|---|---|---|
| Location/type | n | % | n | % |
| Colon | 13 | 21.0 | 15 | 28.3 |
| Bladder | 12 | 19.4 | 8 | 15.1 |
| Lung | 8 | 12.9 | 14 | 26.4 |
| Prostate | 6 | 9.7 | 1 | 1.9 |
| Melanoma | 5 | 8.1 | 1 | 1.9 |
| Breast | 4 | 6.5 | 0 | 0.0 |
| Larynx | 3 | 4.8 | 1 | 1.9 |
| Lymphoma | 2 | 3.2 | 1 | 1.9 |
| Myeloma | 2 | 3.2 | 0 | 0.0 |
| Thyroid | 2 | 3.2 | 0 | 0.0 |
| Stomach | 1 | 1.6 | 5 | 9.4 |
| Leukemia | 1 | 1.6 | 0 | 0.0 |
| Pancreas | 1 | 1.6 | 3 | 5.7 |
| Parotid gland | 1 | 1.6 | 1 | 1.9 |
| Sarcoma | 1 | 1.6 | 0 | 0.0 |
| Uterus/ovaries | 0 | 0.0 | 2 | 3.8 |
| Kidney | 0 | 0.0 | 1 | 1.9 |
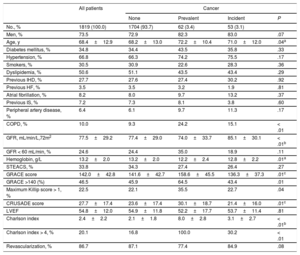
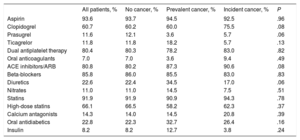
Patients with established cancer tended to be older than noncancer patients and those who developed cancer during follow-up; established cancer was also associated with higher scores on the Charlson, GRACE, and CRUSADE scales and with significantly lower serum hemoglobin (Table 2). No differences were observed in other clinical variables or in ACS severity. The overall revascularization rate was 86.7% and there were no differences between the 3 patient groups; however, drug-eluting stents were used significantly less frequently in patients with prevalent cancer than in other patients (37.7% vs 60.4%; P < .01). There were no between-group differences in medication at discharge (Table 3).
General Characteristics of Patients Classified According to the Presence of Cancer on Admission or Its Development Postdischarge
| All patients | Cancer | ||||
|---|---|---|---|---|---|
| None | Prevalent | Incident | P | ||
| No., % | 1819 (100.0) | 1704 (93.7) | 62 (3.4) | 53 (3.1) | |
| Men, % | 73.5 | 72.9 | 82.3 | 83.0 | .07 |
| Age, y | 68.4±12.9 | 68.2±13.0 | 72.2±10.4 | 71.0±12.0 | .04a |
| Diabetes mellitus, % | 34.8 | 34.4 | 43.5 | 35.8 | .33 |
| Hypertension, % | 66.8 | 66.3 | 74.2 | 75.5 | .17 |
| Smokers, % | 30.5 | 30.9 | 22.6 | 28.3 | .36 |
| Dyslipidemia, % | 50.6 | 51.1 | 43.5 | 43.4 | .29 |
| Previous IHD, % | 27.7 | 27.6 | 27.4 | 30.2 | .92 |
| Previous HF, % | 3.5 | 3.5 | 3.2 | 1.9 | .81 |
| Atrial fibrillation, % | 8.2 | 8.0 | 9.7 | 13.2 | .37 |
| Previous IS, % | 7.2 | 7.3 | 8.1 | 3.8 | .60 |
| Peripheral artery disease, % | 6.4 | 6.1 | 9.7 | 11.3 | .17 |
| COPD, % | 10.0 | 9.3 | 24.2 | 15.1 | < .01 |
| GFR, mL/min/L,72m2 | 77.5±29.2 | 77.4±29.0 | 74.0±33.7 | 85.1±30.1 | < .01b |
| GFR < 60 mL/min, % | 24.6 | 24.4 | 35.0 | 18.9 | .11 |
| Hemoglobin, g/L | 13.2±2.0 | 13.2±2.0 | 12.2±2.4 | 12.8±2.2 | .01a |
| STEACS, % | 33.8 | 34.3 | 27.4 | 26.4 | .27 |
| GRACE score | 142.0±42.8 | 141.6±42.7 | 158.6±45.5 | 136.3±37.3 | .01c |
| GRACE >140 (%) | 46.5 | 45.9 | 64.5 | 43.4 | .01 |
| Maximum Killip score > 1, % | 22.5 | 22.1 | 35.5 | 22.7 | .04 |
| CRUSADE score | 27.7±17.4 | 23.6±17.4 | 30.1±18.7 | 21.4±16.0 | .01c |
| LVEF | 54.8±12.0 | 54.9±11.8 | 52.2±17.7 | 53.7±11.4 | .81 |
| Charlson index | 2.4±2.2 | 2.1±1.8 | 8.0±2.8 | 3.1±2.7 | < .01b |
| Charlson index > 4, % | 20.1 | 16.8 | 100.0 | 30.2 | < .01 |
| Revascularization, % | 86.7 | 87.1 | 77.4 | 84.9 | .08 |
COPD, chronic obstructive pulmonary disease; HF, heart failure; IHD, ischemic heart disease; IS, ischemic stroke; LVEF, left ventricular ejection fraction; STEACS, ST-segment elevation acute coronary syndrome.
Treatment at Discharge in Each Patient Group
| All patients, % | No cancer, % | Prevalent cancer, % | Incident cancer, % | P | |
|---|---|---|---|---|---|
| Aspirin | 93.6 | 93.7 | 94.5 | 92.5 | .96 |
| Clopidogrel | 60.7 | 60.2 | 60.0 | 75.5 | .08 |
| Prasugrel | 11.6 | 12.1 | 3.6 | 5.7 | .06 |
| Ticagrelor | 11.8 | 11.8 | 18.2 | 5.7 | .13 |
| Dual antiplatelet therapy | 80.4 | 80.3 | 78.2 | 83.0 | .82 |
| Oral anticoagulants | 7.0 | 7.0 | 3.6 | 9.4 | .49 |
| ACE inhibitors/ARB | 80.8 | 80.2 | 87.3 | 90.6 | .08 |
| Beta-blockers | 85.8 | 86.0 | 85.5 | 83.0 | .83 |
| Diuretics | 22.6 | 22.4 | 34.5 | 17.0 | .06 |
| Nitrates | 11.0 | 11.0 | 14.5 | 7.5 | .51 |
| Statins | 91.9 | 91.9 | 90.9 | 94.3 | .78 |
| High-dose statins | 66.1 | 66.5 | 58.2 | 62.3 | .37 |
| Calcium antagonists | 14.3 | 14.0 | 14.5 | 20.8 | .39 |
| Oral antidiabetics | 22.8 | 22.3 | 32.7 | 26.4 | .16 |
| Insulin | 8.2 | 8.2 | 12.7 | 3.8 | .24 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers.
Follow-up was achieved in 95.1% of the cohort over a median period of 33.0 months (interquartile range, 18.0-48.0 months). Among the 1731 follow-up participants, 53 de novo cancers were recorded, an incidence of 3.1% (95%CI, 2.4%-4.0%). The most frequent tumor locations were colon, lung, bladder, and pancreas. The mean interval between hospital discharge and the appearance of the incident cancer was 25.0 months (interquartile range, 12.0-56.0 months). The multivariate analysis was adjusted for age, sex, risk factors, previous heart disease, revascularization, ejection fraction, and treatment at discharge; variables showing an association with the appearance of incident cancer were age (sHR, 1.03; 95%CI, 1.01-1.06; P=.01) and current or former smoking (sHR, 2.68; 95%CI, 1.11-6.49; P=.03) (Figure 1). The C statistic for the multivariate model was 0.672.
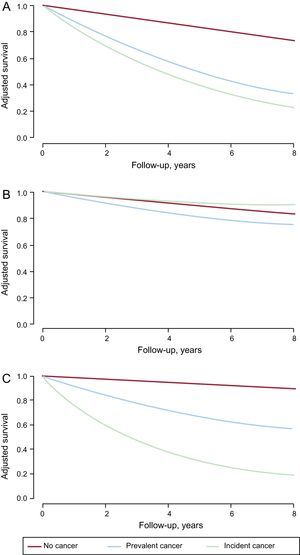
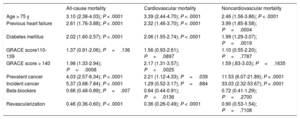
Long-term MortalityOf the 1731 study participants, 280 died during follow-up, corresponding to an all-cause mortality rate of 16.2% (95%CI, 14.5%-18.0%); mortality was much higher in patients with incident cancer (64.2%) and prevalent cancer (40.0%) (Figure 2A); 58.5% of deaths among patients with incident cancer were directly attributed to cancer, whereas among patients with prevalent cancer the figure was 21.8%. Mortality due to a cardiovascular cause was 11.2% (95%CI, 9.8%-12.8%) and was higher among patients with an established cancer at the time of admission for ACS but not among those who developed cancer during follow-up (Figure 2B). Noncardiovascular mortality was elevated in both groups of cancer patients, especially those developing cancer after ACS (Figure 2C). The multivariate analysis showed that prevalent and incident cancer increased the risk of all-cause mortality by 4-fold and 5-fold, respectively (Table 4). Only pre-existing cancer was associated with increased cardiovascular mortality, whereas both cancer categories were strongly associated with noncardiovascular mortality.
Results of Multivariate Analysis
| All-cause mortality | Cardiovascular mortality | Noncardiovascular mortality | |
|---|---|---|---|
| Age > 75 y | 3.10 (2.38-4.03); P < .0001 | 3.39 (2.44-4.70); P < .0001 | 2.46 (1.56-3.86); P < .0001 |
| Previous heart failure | 2.61 (1.76-3.88); P < .0001 | 2.32 (1.46-3.70); P < .0001 | 3.99 (1.85-8.58); P=.0004 |
| Diabetes mellitus | 2.02 (1.60-2.57); P < .0001 | 2.06 (1.55-2.74); P < .0001 | 1.99 (1.29-3.07); P=.0019 |
| GRACE score110-139 | 1.37 (0.91-2.06); P=.136 | 1.56 (0.93-2.61); P=.0897 | 1.10 (0.55-2.20); P=.7787 |
| GRACE score > 140 | 1.98 (1.33-2.94); P=.0008 | 2.17 (1.31-3.57); P=.0025 | 1.59 (.83-3.03); P=.1635 |
| Prevalent cancer | 4.03 (2.57-6.34); P < .0001 | 2.21 (1.12-4.33); P=.039 | 11.53 (6.07-21.89); P < .0001 |
| Incident cancer | 5.37 (3.68-7.84); P < .0001 | 1.29 (0.52-3.17); P=.684 | 33.03 (2.32-53.67); P < .0001 |
| Beta-blockers | 0.66 (0.48-0.89); P=.007 | 0.64 (0.44-0.91); P=.0136 | 0.72 (0.41-1.29); P=.2700 |
| Revascularization | 0.46 (0.36-0.60); P < .0001 | 0.36 (0.26-0.49); P < .0001 | 0.90 (0.53-1.54); P=.7108 |
The data from this ACS patient cohort reveal a nonnegligible cancer prevalence and incidence and show that established and newly emerging cancers are both associated with very poor prognosis. Cancer prevalence and incidence in ACS patients has received very little attention. However, the clinical characteristics and incidence of complications in the present cohort were similar to those recorded in other registries, both national5,9–11,16 and international.3,4,17 This would suggest that the results obtained with this cohort, with more than 100 cancer cases, are likely to be representative of real clinical practice.
Heart disease and cancer share many risk factors, and both diseases frequently occur in the same patient. Both diseases are associated with smoking, physical inactivity, and poor diet,3,5,18–20 which are currently considered major health problems. Data from Spain predict a more than 30% increase in cancer incidence compared with previous decades, essentially as a consequence of population aging and changes in risk factors.21 The present study shows that current or former smoking is one of the main risk factors for cancer development after ACS, underlining the importance of smoking as a risk factor for both diseases. Moreover, 2 of the most frequent cancers in the series were lung and bladder cancers, both of which are directly linked to tobacco consumption. Smoking is a major risk factor for ACS,19,22 especially in the young.23 The INTERHEART study demonstrated that smokers have an almost 3-fold higher risk of an infarction than nonsmokers (odds ratio [OR], 2.87; 95%CI, 2.58-3.19).24 The same study also showed that former smokers who had stopped smoking less than 3 years previously had an infarction risk intermediate between that of smokers and nonsmokers (OR, 1.87; 95%CI, 1.55-2.24) and an almost 10-year residual risk of ACS. In the present study, we did not evaluate smoking abstinence during follow-up; however, national data indicate that more than 15% of patients are still smokers more than a year after presenting with ACS.25 Moreover, smokers tend to be less physically active than nonsmokers and show poorer adherence to dietary recommendations for cardiovascular health.20 The association between smoking and the appearance of incident cancer is consistent with knowledge of the dangers of tobacco use and underlines the need to include antismoking measures across the spectrum of cardiovascular prevention; recent studies have reported increases in the percentage of smokers in both primary and secondary prevention.26,27
The higher cardiovascular mortality rate in patients with established cancer may be related to the lower rates of coronary revascularization and drug-eluting stent implantation in this group. This interpretation is supported by the normal cardiovascular mortality rate in ACS patients who were cancer-free on admission but who developed cancer during follow-up. The presence of comorbidities complicates decisions about revascularization and especially about the use of antiplatelet therapy and drug-eluting stents, and this concern is especially important for comorbidities that increase bleeding incidence. Coronary revascularization has been shown to be fully effective in patients with more comorbidities28; however, prevalent and incident cancers are strongly associated with a higher incidence of gastrointestinal bleeding.29,30 The Spanish Society of Cardiology consensus document on Cardio-Onco-Hematology specifies ischemic heart disease as a risk factor for the development of cardiotoxicity in patients undergoing cancer therapy.8
Previous studies did not provide a detailed analysis of cancer incidence and prognosis associated with ACS, and, to our knowledge, the present study is the first to analyze the types and prognoses of prevalent and incident cancers in ACS patients. Previous long-term follow-up data from the SYNTAX study of chronic ischemic heart disease patients revealed noncardiovascular mortality rates of 4.3% for those receiving percutaneous revascularization and 5.3% for those undergoing surgery, with cancer mortality rates of 2.2% and 2.4%, respectively.31 The SYNTAX study did not distinguish between prevalent cancer at the time of inclusion and incident cancers developing during follow-up and did not identify cancer as an independent predictor of noncardiovascular mortality. In the RECALCAR registry, the prevalence of malignant tumors among patients admitted for ACS was 2.77%, and prevalent cancer was associated with higher in-hospital mortality (OR, 2.26; 95%CI, 1.99-2.55).16 In contrast, a recent Spanish single-center study reported a cancer prevalence of 7.7% in a large population of patients admitted for ACS; however, follow-up in this study was limited to the analysis of major cardiovascular complications. In the large CREDO-Kyoto AMI registry (Coronary Revascularization Demonstrating Outcome study in Kyoto Acute Myocardial Infarction), comparison of short-term and long-term mortality revealed a prominence of cardiac causes during the first 6 months postinfarction (8.0%); however, at 5 years’ follow-up, noncardiac causes were prominent, with the mortality rate almost double that for cardiac causes (8.5% vs 4.6%).32 The CREDO-Kyoto AMI study did not report cancer mortality. The most frequent incident cancers in our cohort were lung, colon, and bladder cancers. This finding does not fully coincide with published data that include breast and prostate cancers among the most frequent tumors1; however, this discrepancy may directly reflect the focus of the present study on ACS survivors with a mean age close to 70 years. Moreover, our data clearly demonstrate a worse prognosis for ACS patients who either have or who later develop cancer and reveal the need for highly specific treatment and follow-up for these patients. The survival curves for ACS patients with prevalent and incident cancer are very similar, suggesting that these patient groups are distinguished only by the timing of cancer development.
LimitationsThe main limitation of the current report is that it is an observational single-center study. Nonetheless, the clinical characteristics and incidence of complications during follow-up are very similar to those reported in previous publications,5,9–11,16,17 suggesting that the results are likely valid and representative of routine clinical practice.
CONCLUSIONSA low proportion of patients admitted for ACS had a history of cancer; however, fewer than half of these patients were cancer-free on admission, and these patients had a very poor postdischarge prognosis. Moreover, the postdischarge cancer incidence was 3.4%, and patients with incident cancer also had a very high mortality rate. The main risk factor for postdischarge cancer development was tobacco use, indicating that measures aimed at smoking prevention and cessation could be especially beneficial in patients with or at risk of ACS. Given the very poor prognosis of patients with both ACS and cancer, we believe that a multidisciplinary approach is needed to improve treatment, quality of life, and prognosis.
CONFLICTS OF INTERESTNone declared.
- –
Ischemic heart disease and cancer are the main causes of death worldwide.
- –
Cancer and ischemic heart disease share many of the same risk factors.
- –
The presence of comorbidities worsens the prognosis of ACS patients.
- –
In a population of patients admitted for ACS, 3.4% had a history of cancer and 3.1% developed cancer during follow-up.
- –
The most frequent incident cancers after ACS were colon, lung, and bladder cancers.
- –
Current or former smoking was the primary risk factor for the development of cancer after ACS.
- –
All-cause mortality among ACS patients with cancer was higher than 40%.