The proportion of elderly patients undergoing primary angioplasty is growing. The present study describes the clinical profile, procedural characteristics, outcomes, and predictors of outcome.
MethodsA 31-center registry of consecutive patients older than 75 years treated with primary angioplasty. Clinical and procedural data were collected, and the patients underwent clinical follow-up.
ResultsThe study included 3576 patients (39.3% women, 48.5% with renal failure, 11.5% in Killip III or IV, and 29.8% with>6hours of chest pain). Multivessel disease was present in 55.4% and nonculprit lesions were additionally treated in 24.8%. Radial access was used in 56.4%, bivalirudin in 11.8%, thromboaspiration in 55.9%, and drug-eluting stents in 26.6%. The 1-month and 2-year incidences of cardiovascular death were 10.1% and 14.7%, respectively. The 2-year rates of definite or probable thrombosis, repeat revascularization, and BARC bleeding>2 were 3.1%, 2.3%, and 4.2%, respectively. Predictive factors were diabetes mellitus, renal failure, atrial fibrillation, delay to reperfusion>6hours, ejection fraction<45%, Killip class III-IV, radial access, bivalirudin, drug-eluting stents, final TIMI flow of III, and incomplete revascularization at discharge.
ConclusionsNotable registry findings include frequently delayed presentation and a high prevalence of adverse factors such as renal failure and multivessel disease. Positive procedure-related predictors include shorter delay, use of radial access, bivalirudin, drug-eluting stents, and complete revascularization before discharge.
Keywords
The population of Western countries, particularly that of Spain, is aging markedly, with a parallel increase in the proportion of elderly patients admitted with ST elevation acute coronary syndrome.1 Primary angioplasty (PA) is the treatment of choice if it can be performed within an appropriate time frame. Although advanced age is associated with worse infarction prognosis, PA continues to be the best reperfusion strategy for elderly patients.2,3
Nonetheless, reperfusion therapy was used in few elderly patients in Spain until recently and, when applied, PA was less frequently chosen than other reperfusion strategies.4
Various registries have associated a worse prognosis with increased age among patients undergoing PA5–7 but, given the exclusion of these patients from PA studies or their very low representation, as well as the increased use of PA in the elderly in Spain, there is a notable scarcity of information on the clinical profile of patients older than 75 years treated with PA in Spain, procedural characteristics, in-hospital and long-term outcomes, and predictors of outcome. Consequently, there is no evidence on the value of the different PA strategies in this patient group.
We undertook the present multicenter registry first to determine PA characteristics and outcomes in patients older than 75 years in Spain and, second, to evaluate the clinical and procedural predictors of outcome.
METHODSThe present study concerned a retrospective registry of 31 centers distributed throughout Spain. This registry is supported by the Hemodynamics Section of the Spanish Society of Cardiology and is part of the ESTROFA (Estudio Espanol Sobre Trombosis de Stents Farmacoactivos [Spanish Thrombosis in Drug-eluting Stents Study]) series of trials.
PopulationEach center enrolled a strictly consecutive series of patients aged >75 years who underwent PA due to ST-elevation acute coronary syndrome and were treated with bare-metal stents (BMS) or new-generation drug-eluting stents (DES). The sample sizes of the series of each center depended on the number of admissions to that center of patients with ST elevation acute coronary syndrome but included only patients with at least 1 year of follow-up from the start date of the study; patient enrollment was retrospective and strictly consecutive. The retrospective inclusion period ran from 2006 (at the earliest) to 2013, although most patients were enrolled in the most recent period (2010-2013).
Additionally, this study also included patients older than 75 years from the EXAMINATION trial, also performed in Spain.8
We did not apply clinical or angiographic exclusion criteria. The procedures were performed according to the preferences of each operator and each institution. The clinical, angiographic, procedural, and follow-up information was collected in a specifically designed study database.
Event Analysis and DefinitionsPatients were followed up by reviewing local databases, hospital or unit registries, and medical records, as well as via personal or familial telephone contact if necessary. The database was analyzed by 2 researchers in the coordinating center.
The events identified by the researchers were adjusted to the definitions provided; nonetheless, to guarantee their homogeneous application, the final event adjudication was performed by the coordinating center based on the data provided. Additional information was requested when necessary.
The following major adverse cardiac events were defined: a) mortality, as all-cause death; b) cardiovascular death, as death due to cardiovascular causes, including unexplained sudden death; c) myocardial infarction if the event fulfilled certain criteria meeting the third definition of infarction of the European Society of Cardiology and the American College of Cardiology; d) repeat revascularization, including any revascularization procedure in previously treated culprit lesions; e) stent thrombosis, classified according to the Academic Research Consortium system9; and f) bleeding, scored according to the classification of the Bleeding Academic Research Consortium (BARC).10 Bleeding was considered major if it had a BARC score>2.
Statistical AnalysisContinuous variables are presented as mean±standard deviation and discrete variables as percentages. Continuous variables were compared using the t test if they followed a normal distribution, determined using the Kolmogorov-Smirnov test; otherwise, Wilcoxon tests were used. Discrete variables were compared with the chi-square or Fisher exact tests as necessary. A Cox proportional risk analysis was used to establish predictors of events during follow-up. The model included all variables achieving P<.02 in the univariable analysis, which included a hospital center variable. P<.05 was considered statistically significant. All statistical analyses were performed with SPSS software version 19 for Windows.
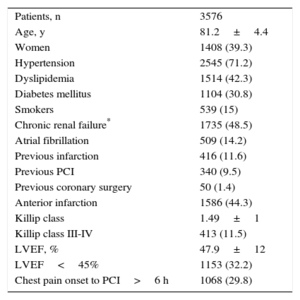
RESULTSA total of 3576 patients were included from 31 centers. The patients’ clinical and procedural characteristics are shown in Table 1 and Table 2. Women comprised 39.3% of the series; chronic renal failure affected half of the patients; despite the age of the patients, 11.5% were in Killip class III-IV, and there was a delay from symptom onset to reperfusion >6hours in 29.8%. The procedural and angiographic data showed that 55.4% of the patients had multivessel disease; other lesions were treated in addition to the culprit lesion in 24.8% of patients, whether in the acute phase (53%) or as another procedure during hospitalization (47%). A third of the patients was discharged with incomplete angiographic revascularization, defined as the presence of stenosis >50% in nonrevascularized vascular segments with a reference diameter ≥ 2mm.
Clinical Characteristics
| Patients, n | 3576 |
| Age, y | 81.2±4.4 |
| Women | 1408 (39.3) |
| Hypertension | 2545 (71.2) |
| Dyslipidemia | 1514 (42.3) |
| Diabetes mellitus | 1104 (30.8) |
| Smokers | 539 (15) |
| Chronic renal failure* | 1735 (48.5) |
| Atrial fibrillation | 509 (14.2) |
| Previous infarction | 416 (11.6) |
| Previous PCI | 340 (9.5) |
| Previous coronary surgery | 50 (1.4) |
| Anterior infarction | 1586 (44.3) |
| Killip class | 1.49±1 |
| Killip class III-IV | 413 (11.5) |
| LVEF, % | 47.9±12 |
| LVEF<45% | 1153 (32.2) |
| Chest pain onset to PCI>6 h | 1068 (29.8) |
LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Data are presented as mean±standard deviation or No. (%).
Procedure Characteristics
| Patients, n | 3576 |
| Radial access | 2016 (56.4) |
| Baseline TIMI flow | 0.65±1 |
| Baseline TIMI flow 0-1 | 2779 (77.7) |
| Diseased vessels | 1.84±0.8 |
| Multivessel disease | 1982 (55.4) |
| Treated lesions | 1.23±0.5 |
| Number of stents implanted | 1.28±0.58 |
| PCI of NC lesions in acute phase | 475 (13.2) |
| PCI of NC lesions in another procedure during the hospitalization | 416 (11.6) |
| Patients with incomplete revascularization at discharge* | 1264 (35.3) |
| Stent length, mm | 21.3±7.6 |
| Stent diameter, mm | 3±0.48 |
| NC lesions treated (acute) | 0.19±0.47 |
| NC lesions treated (deferred) | 0.2±0.56 |
| Untreated NC lesions | 0.73±1.1 |
| UFH alone | 2323 (64.9) |
| UFH+anti-GPIIb/IIIa | 830 (23.2) |
| Bivalirudin alone | 416 (11.6) |
| Bivalirudin+anti-GPIIb/IIIa | 7 (0.2) |
| Thromboaspiration | 2000 (55.9) |
| DES | 952 (26.6) |
| Final TIMI flow | 2.85±0.5 |
| Final TIMI flow III | 3295 (92.1) |
| DAPT>6 mo | 2327 (65) |
| Ticagrelor/prasugrel | 106 (2.9) |
| Oral anticoagulation | 453 (12.6) |
Anti-GPIIb/IIIa, glycoprotein IIb/IIIa inhibitor; DAPT, dual antiplatelet therapy; DES, drug-eluting stents; NC, nonculprit; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; UFH, unfractionated heparin.
Data are presented as mean±standard deviation or No. (%).
Slightly more than half of the PA procedures were performed via the radial approach. In addition, most patients were anticoagulated with unfractionated heparin (1 of every 4 patients was treated with a glycoprotein IIb/IIIa receptor inhibitor, generally abciximab), half of the patients underwent thromboaspiration, and DES were implanted less frequently (26.6%) than BMS.
In total, 2.8% of patients were lost to follow-up, typically because they were nationals of other countries. The events described in Table 3 were recorded during a median clinical follow-up of 25 months (interquartile range, 14-38 months). The 1-month incidence of death was 12.2%, which doubled at 2 years. The most common cause of death was clearly cardiovascular in the short-term, constituting 83% of deaths in the first month but decreasing to 61% at 2 years. The incidence of stent thrombosis was 3.1%, with almost equal distribution between the subacute and late phases. At 2 years, major bleeding (BARC >2) was more frequent than stroke or repeat revascularization.
Incidence of Major Adverse Events During Follow-up
| 1 mo | 2 y | |
|---|---|---|
| Patients, n | 3576 | 1895 |
| Deaths, total | 437 (12.2) | 459 (24.2) |
| Cardiovascular death | 362 (10.1) | 279 (14.7) |
| Cardiovascular death+infarction | 383 (10.7) | 318 (16.8) |
| Cardiovascular death+infarction+RR | 385 (10.7) | 326 (17.2) |
| Definite or probable stent thrombosis | 57 (1.6) | 59 (3.1) |
| RR | 32 (0.9) | 44 (2.3) |
| BARC bleeding>2 | 18 (0.5) | 79 (4.2) |
| Stroke | 22 (0.6) | 42 (2.2) |
BARC, Bleeding Academic Research Consortium; RR, repeat revascularization.
Data are presented as No. (%). Events were collected from the total group of 3576 patients with 1-month follow-up and from the subgroup of 1895 patients with 2-year follow-up.
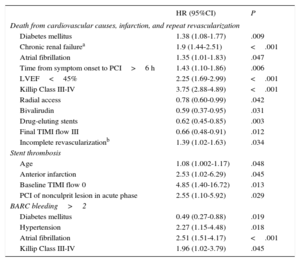
Independent predictors of adverse events are shown in Table 4. The main predictors of cardiovascular death, infarction, and repeat revascularization were well-known adverse factors, such as renal failure, diabetes mellitus, and advanced Killip class, as well as less common procedural variables with protective effects, such as the use of the radial approach, bivalirudin, or DES, and some adverse factors, such as delayed presentation and incomplete revascularization at discharge. Thrombosis predictors were also identified, notably anterior infarct location and revascularization of nonculprit lesions in the acute phase, as well as those of major bleeding. Some factors promoting the latter complication were highly prevalent in this population, such as hypertension and atrial fibrillation.
Independent Predictors of Adverse Events
| HR (95%CI) | P | |
|---|---|---|
| Death from cardiovascular causes, infarction, and repeat revascularization | ||
| Diabetes mellitus | 1.38 (1.08-1.77) | .009 |
| Chronic renal failurea | 1.9 (1.44-2.51) | <.001 |
| Atrial fibrillation | 1.35 (1.01-1.83) | .047 |
| Time from symptom onset to PCI>6 h | 1.43 (1.10-1.86) | .006 |
| LVEF<45% | 2.25 (1.69-2.99) | <.001 |
| Killip Class III-IV | 3.75 (2.88-4.89) | <.001 |
| Radial access | 0.78 (0.60-0.99) | .042 |
| Bivalirudin | 0.59 (0.37-0.95) | .031 |
| Drug-eluting stents | 0.62 (0.45-0.85) | .003 |
| Final TIMI flow III | 0.66 (0.48-0.91) | .012 |
| Incomplete revascularizationb | 1.39 (1.02-1.63) | .034 |
| Stent thrombosis | ||
| Age | 1.08 (1.002-1.17) | .048 |
| Anterior infarction | 2.53 (1.02-6.29) | .045 |
| Baseline TIMI flow 0 | 4.85 (1.40-16.72) | .013 |
| PCI of nonculprit lesion in acute phase | 2.55 (1.10-5.92) | .029 |
| BARC bleeding>2 | ||
| Diabetes mellitus | 0.49 (0.27-0.88) | .019 |
| Hypertension | 2.27 (1.15-4.48) | .018 |
| Atrial fibrillation | 2.51 (1.51-4.17) | <.001 |
| Killip Class III-IV | 1.96 (1.02-3.79) | .045 |
95%CI, 95% confidence interval; BARC, Bleeding Academic Research Consortium; HR, hazard ratio; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Model adjustment with P<.001.
Primary angioplasty performed within an acceptable time frame is the best reperfusion strategy for older patients,2,3 although their prognosis is still worse than that of younger patients.5–7,11–13 Acceptable results have even been reported for nonagenarians.14 Nonetheless, until recently, PA was infrequent in the elderly population in Spain.4 No large and specific Spanish registries have described the characteristics and outcomes of contemporaneous PA in these patients.
In addition, there is little evidence or knowledge of the outcomes of the alternative PA strategies in this population group. Because patients aged 75 to 80 years have been excluded from PA trials or are poorly represented,15 PA procedures in these patients are undertaken based on evidence obtained from the general population or the experience of the operators themselves.
Analysis of the profile of patients older than 75 years treated with PA in recent years in Spain revealed a higher proportion of women vs series containing the general population, a high prevalence of renal failure, and a considerably delayed presentation (> 6 hours) in almost a third of patients. This latter finding is important because a delayed presentation >6hours was identified as a predictor of events. In addition, despite the doubts surrounding the viability of PA in elderly patients with cardiogenic shock, 11.5% were in Killip class III-IV.
Regarding procedural aspects, the radial approach was used in slightly more than half of the patients and was independently associated with fewer adverse events. Radial access is superior to femoral access in PA16–19 but this approach can be more difficult in elderly patients, particularly women, and is associated with higher failure rates and access site crossover, with potential impacts on clinical outcomes.20 However, elderly patients also have a higher risk of bleeding with femoral access.
Bivalirudin use as antithrombotic therapy was clearly minimal (11.8%) but was an independent predictor of positive outcomes. Notably, bivalirudin was not a predictor of stent thrombosis or BARC bleeding >2, probably due to the frequently delayed incidence of these events, particularly bleeding, vs the initial high mortality rate. Although there is no evidence and, thus, no specific recommendations for these patients, bivalirudin use would a priori be more attractive in the elderly population due to their higher bleeding risk. However, controversy surrounds the advantages of this drug over unfractionated heparin, in light of the latest trials and a meta-analysis indicating that the lower bleeding risk with bivalirudin ceases to be significant vs low levels glycoprotein IIb/IIIa inhibitors combined with unfractionated heparin.21–23 In addition, in 2 studies, the superiority of bivalirudin was age-dependent and was no longer evident in young patients, although another study found the opposite.24–26
Thromboaspiration was used in slightly more than half of the series but was not a predictor of events. Systematic application of this strategy failed to improve prognosis.27,28 Nonetheless, its selective use might offer some advantages, particularly for the highest-risk populations, such as elderly patients, because worse reperfusion after PA is an additional determinant of their poor prognosis.29
The least implanted stents were DES, but their use was an independent predictor of a positive effect. The use of DES in myocardial infarction has been questioned for various reasons, possibly explaining their low rate of use in this context compared with their general use. The incidence of symptomatic restenosis in culprit lesions of ST-elevation infarction is lower than that of other lesions due to the variable loss of myocardial mass and is even worse in this population who are less physically active. Second, although late thrombosis is less frequent with newer-generation DES, it could be a problem in these patients with a higher risk of major and minor bleeding, nonelective surgical interventions, and therapeutic adherence failures. The single randomized trial that has evaluated BMS and DES in elderly patients obtained positive results with DES but excluded patients with PA.30 In the context of PA, a substudy of the HORIZONS-AMI trial failed to show a significant benefit from paclitaxel DES over BMS in patients older than 70 years.31 A subanalysis of the EXAMINATION trial centered on elderly patients; the analysis included 132 patients treated with BMS and 113 treated with everolimus DES and failed to show the benefits of DES over BMS.32
The role of complete revascularization is important. In our population, 55.4% had multivessel disease and, although a certain percentage of patients underwent revascularization of nonculprit lesions in the acute phase or as a subsequent procedure during admission, 35.3% had significant residual disease at discharge. This variable predicted adverse events. This finding is in line with the benefit observed with complete revascularization in trial meta-analyses.33,34 Nonetheless, in this registry, treatment of nonculprit lesions in the acute phase was a predictor of stent thrombosis, providing support for the strategy of complete revascularization via deferred procedures.
The 1-month mortality seen in our registry (12.2%) was clearly higher than that observed in the general population but is within the range published by other registries of this population.5,6,12,13 The 2-year mortality rate doubled to reach 24.2%, although with a notable contribution of noncardiovascular mortality, differentiating this population series from that of the general population. Notably, the incidence of cardiovascular mortality from the first month to 2 years was not so high (4.6%). Similarly, a large Swedish registry5 found that elderly infarction survivors even have a certain advantage over the general population for adverse cardiac events in the subsequent year, probably due to the strict initial selection of patients during the acute phase.
The incidence of repeat revascularization was low, given that most patients received BMS, and is lower than that observed in series of elderly patients outside the context of PA.29 The 2-year incidence of definitive or probable thrombosis was 3.1%, with a similar distribution between subacute and late phases. Major bleeding was more frequent than thrombosis, repeat revascularization, and stroke and occurred slightly more sporadically. The incidence of major bleeding was linked to highly prevalent factors, such as hypertension and atrial fibrillation, and was frequently associated with oral anticoagulation. Notably, DES were linked to longer duration of dual antiplatelet therapy but were not associated with a higher incidence of major bleeding, indicating adequate patient selection by the operator (eg, preferential use of BMS in patients requiring oral anticoagulants).
LimitationsThis is a retrospective multicenter registry and, although patients were strictly consecutively recruited from each center, data collection and follow-up were not as accurate and uniform as they would have been with a prospective design. Due to the retrospective design, it was not possible to record medical therapy adherence and its eventual relationship with ischemic and hemorrhagic events. We were also unable to estimate the proportion of patients older than 75 years with infarction enrolled in each center and the proportion of patients who underwent angiography but not PA, whether due to a lack of indication or death or because the angioplasty was performed with just a balloon. The purpose of the registry was to analyze a population who underwent angiography and PA (with a final diagnosis of ST elevation infarction with no involvement of other conditions) whose treatment could be completed according to the usual care (stent implantation).
The results of the predictor analysis might have been somewhat affected by hidden biases, such as comorbidities omitted from the databases or biological frailty, which could in turn be linked to some therapeutic strategies more than others.
CONCLUSIONSIn this multicenter registry of PA in patients older than 75 years, we observed a frequent delay in presentation and a high prevalence of adverse factors such as renal failure. The 1-month and 2-year mortality rates were 12.2% and 24.2%, respectively. Radial access and thromboaspiration were used in half the patients, as well as bivalirudin and DES in a minority. The following positive procedure-related predictors were identified: shorter delay, use of radial access, bivalirudin, and DES, and complete revascularization before discharge.
CONFLICTS OF INTERESTNone declared.
- –
Although PA has been recommended as the reperfusion strategy of choice for more than 10 years, as long as it is performed within an appropriate time frame, the procedure has only recently become common in Spain and is still used irregularly.
- –
In older populations, which are growing, this reduced use of PA is even more marked.
- –
In addition, these patients have been excluded from PA trials or are poorly represented.
- –
Thus, there is little information on the clinical and angiographic profile, procedural characteristics, short- and long-term outcomes, prognostic predictive factors, and the value of alternatives PA strategies in the population aged>75 years.
- –
Our study analyzed a large contemporaneous series of patients older than 75 years who underwent PA in Spain.
- –
We characterized the patients’ profiles, procedural characteristics, and 1-month and 2-year clinical outcomes.
- –
Predictors of adverse events were identified, including procedure-related variables that are thus modifiable.