At the beginning of the century, determination that primary percutaneous coronary intervention (PPCI) was the best reperfusion strategy in the setting of ST-segment elevation acute coronary syndrome (STEACS)1 led to the implementation of primary care networks for acute myocardial infarction. The objective of these networks was to quickly identify and treat patients with this entity. Along with pharmacological advances, this approach has noticeably improved the short- and long-term prognoses of patients with STEACS.2,3 However, the impact has not been equal among all age groups, with less benefit in more elderly patients.4
Traditionally, the prognosis of a condition is determined using cumulative survival curves. However, this approach has 3 major limitations. First, a prognosis is not static but dynamic, exhibiting considerable changes over time, especially if the patient survives a specific timeframe. Second, cumulative survival rates do not distinguish between which mortality percentage is due to the condition and which is due to other causes. This is especially important for older patients, who may have causes of death that compete with those of the condition of interest. In these patients, the cumulative survival curves offer a much more pessimistic view of the disease. In addition, this focus does not allow for evaluation of the net impact of an intervention or treatment on vital prognosis. The third limitation is that the prognosis of any condition must be compared with a standard to ascertain whether it is good or bad. Our comparisons tend to be temporal, with entities compared in terms of survival vs the past or based on an additional treatment that was not previously available. However, from patients’ perspective, the most important question is how their prognosis compares with that of someone in the same situation without the illness. To put it another way, what life expectancy they would have vs a person with the same age, sex, and characteristics but without the disease. This comparative framework is what really establishes the impact of an illness and allows assessment of its true prognosis.
Various approaches have been proposed to overcome these difficulties. One of the most interesting is calculation of relative survival (RS), which permits evaluation of the impact of a specific entity on affected patients. This is especially useful in observational studies, where it is very difficult to establish the different causes of death. To do this, the cumulative survival curve of individuals with a disease (observed survival) is compared with that of a disease-free comparator group in the general population (expected survival). This ratio is known as relative survival (RS). This comparison offers considerable advantages. It allows us to calculate the excess mortality rate of patients with a disease while isolating it from other unknown causes of death that are shared with the general population. It enables comparison quantification of the impact of the treatments used. And, finally, it helps to establish if there is any point at which the life expectancy of patients is the same as that of their healthy counterparts.
When the RS is being calculated, there are 2 problems that can distort results in opposing directions and therefore limit its accuracy. The first arises because the population survival tables for comparing patients with the general population are usually only adjusted for age, sex, and geographical location. There are many more covariables in the study population that are likely to predispose it to the disease that are not adjusted for in the general population. This is the case with cardiovascular disease risk factors, for example. Without this adjustment, the survival rate of the general population tends to be overestimated and, as a result, the RS is underestimated. On the other hand, if the condition being studied is also highly prevalent in the general population, some of the observed mortality will be shared by the population under study. Therefore, the survival rate in the general population will be diminished by deaths attributable to the disease of interest. If it is excluded, the survival rate will be higher, which is to say that the survival of the general population will be underestimated. Accordingly, when the RS is calculated, it tends to be overestimated. Various solutions have been proposed to correct these errors.5
Despite the above limitations, analysis of RS and the calculation of excess mortality have been widely used for some time, especially in the field of oncology.6,7 With these metrics, oncologists have been able to determine the timeframe in which a patient who has had cancer can be considered “cured”. This is the moment at which their life expectancy is the same as that of the matched, disease-free, general population. This valuable approach has also been proposed for evaluating cardiovascular diseases.8
This is precisely the context that must frame the SurviSTEMI study, recently published by Pascual et al.9 in Revista Española de Cardiología. The authors analyzed 1722 patients with STEACS who underwent PPCI reperfusion between March 2014 and March 2020. The main objective was to determine if the survival rate was similar in these patients and in a respective comparator population of the same age, sex, and geographical location, stratified into 2 subgroups according to age: < 65 and ≥ 65 years. A subanalysis was also carried out on octogenarian patients. Tables from the Spanish National Institute of Statistics (INE) were used to calculate the expected survival of the population. Observed survival was calculated with the actuarial method: the ratio between the 2 survival rates enabled the calculation of RS and excess mortality (1 – RS). The authors performed these calculations at the 5-year follow-up. However, given that the average follow-up time was 34 months (2.8 years), the inaccuracy increased for the 4- and 5-year estimates and we have thus concentrated on the results of the first 3 years.
The SurviSTEMI findings can be summarized in 3 points:
- 1.
In patients with STEACS and PPCI, independently of age, RS was lower and excess mortality higher than in their comparators in the background population.
- 2.
This excess mortality was more accentuated in the elderly age group and was particularly concentrated in the first year; after this time, it disappeared.
- 3.
Regarding the RS and excess mortality of patients who survived the first 30 days, both metrics were observed to be similar to those of the general population with the same age, sex, and geographical area.
These same results were also obtained in other studies, with the same findings almost systematically repeated. Patients with STEACS treated with PPCI who survived the first month, independently of age, have an excellent prognosis in terms of RS and excess mortality, comparable to that of the general population.10,11 In fact, after the first year, the principal causes of mortality are noncardiovascular in this group of patients.12
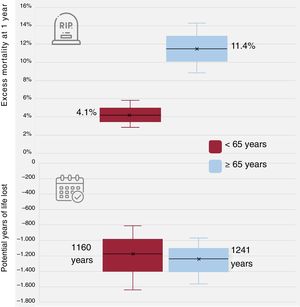
One pertinent aspect of the excess mortality calculation is that it also allows us to quantify the potential years of life lost (PYLLs). The excess mortality during the first year observed in the SurviSTEMI study9 was markedly higher in older patients: 4.12% in those younger than 65 years, 11.36% in the group ≥ 65 years, and 15.65% in those ≥ 80 years. However, these differences do not permit us to establish the cost in terms of diminishing life expectancy. Cardiovascular disease is not only one of the main causes of death, especially in western countries, but is also a cause of premature mortality. Estimates for 2017 put the number of PYLLs at around 165 million.13 We calculated the PYLLs for the age groups < 65 (average, 54.21) years and ≥ 65 (average, 75) years. To do this, we used the life expectancy tables of the INE.14 The average age and percentage of women in each of these groups were also considered. As can be seen in figure 1, even though the excess mortality was almost 3 times higher in the elderly age group, the number of PYLLs during the first year was similar in the 2 groups. Neither RS nor excess mortality measures this aspect, although it can be deduced from their data. The calculation of PYLLs gives more information on the true cost of premature death from a condition.
The SurviSTEMI study is an excellent example of the application of RS to the field of cardiology. This approach represents a change in perspective that produces more useful information than that derived from traditional cumulative survival rates. It allows us to compare the prognosis of an entity with respect to the disease-free background population. It is also capable of measuring the impact of different treatments on survival. From patients’ perspective, it answers those crucial questions about their life expectancy and from which time their risk is the same that of as their healthy counterparts. Finally, it permits us to measure impact in terms of life lost through PYLLs.
In conclusion, the study by Pascual et al.9 demonstrates that, for the specific group of STEACS patients treated with PPCI who survive the first month, the life expectancy prognosis is excellent in relation to the general population of the same age and sex. This prognosis is worse for older patients, even though, in terms of PYLLs, the cost is similar to that of people 20 years younger. These results highlight the enormous cost of premature death.
FUNDINGThe authors have not received any funding for this work.
CONFLICTS OF INTERESTNone declared.