Malnutrition has been shown to affect clinical outcomes in patients with heart failure. The aim of this study was to analyze the impact of preoperative nutritional status assessed by the nutritional risk index (NRI) on the prognosis of patients with a continuous-flow left ventricular assist device (cf-LVAD).
MethodsWe performed a retrospective study of 279 patients who underwent cf-LVAD implantation between 2009 and 2015 in our center. Preoperative NRI was calculated and the patients were followed-up for 1 year. The association between preoperative NRI and postoperative clinical events was analyzed using multivariable logistic regression.
ResultsThe prevalence of severe (NRI <83.5), moderate (83.5 ≤ NRI <97.5) and mild (97.5 ≤ NRI <100) nutritional risk was 5.4%, 21.5%, and 9.3%. Mortality rates 1 year after cf-LVAD implantation in these 3 categories were 53.3%, 31.7%, 23.1% vs 18.0% (P <.001) in patients with a normal IRN. A normal preoperative NRI value was an independent predictor of lower risk of death from any cause during follow-up (aHR per 1 unit, 0.961; 95%CI, 0.941-0.981; P <.001) was and a predictor for a lower risk of postoperative infections (aOR, 0.968; 95%CI, 0.946-0.991; P=.007), respiratory failure (aOR, 0,961; 95%CI, 0.936-0.987; P=.004), and right heart failure (aOR, 0.963; 95%CI, 0.934-0.992; P=.014).
ConclusionsMalnourished patients are at increased risk for postoperative complications and death after cf-LVAD implantation. Assessment of nutritional risk could improve patient selection and the early initiation of nutritional support.
Keywords
Ventricular assist device implantation has become a fundamental tool in the treatment of end-stage heart failure (HF). Its use as a bridge to transplant has also become significantly more popular, particularly in countries with long transplant waiting lists. Indeed, a quarter of patients on the heart transplant waiting list in the United States has one of these devices.1 Its use as destination therapy has also undergone exponential growth.1
The large size and short durability of pulsatile flow devices2 have prompted the development of new continuous-flow centrifugal and axial flow pumps with improved life expectancy.3,4 However, despite fewer complications, they continue to be the Achilles heel of this therapy. The appropriate selection of candidates for ventricular assist device therapy is essential to reduce complications and improve results.
Heart failure is commonly associated with weight loss that, in advanced stages, culminates in cardiac cachexia.5 The pathogenesis of the cachexia is unclear and there appear to be several contributing mechanisms. On the one hand, HF fosters an increase in the catabolic state and, on the other hand, it seems that there is an inflammatory and neurohormonal activation that favors the loss of muscle mass and induces anorexia.5–9
Two classic markers of malnutrition–low body mass index10 and hypoalbuminemia11–are correlated with increased mortality in this population. However, neither of these 2 parameters is a reliable indicator of the nutritional status of patients with HF because both can be significantly altered by the disease itself. Their values can be affected by the HF-related inflammatory status, fluid overload, and liver and kidney dysfunction.11 In addition, changes due to water retention can significantly modify body mass index.12
The nutritional risk index (NRI) is a nutritional assessment score that has been widely used in recent years.13 It is simple to apply and has considerable prognostic value for different patient profiles. The NRI is based on objective measurements and is calculated as (1.5 × serum albumin [g/L]) × 41.7 × (current body weight/ideal body weight [IBW]). An NRI > 100 indicates that there is no evidence of malnutrition; 97.5 to 100, mild malnutrition; 83.5 to 97.5, moderate malnutrition, and < 83.5, severe malnutrition. The NRI has been validated as an independent predictor of mortality and adverse clinical events in a broad spectrum of patients with HF.13–15
The aim of this study was to analyze the prognostic value of preoperative NRI in patients with advanced HF who underwent continuous-flow left ventricular assist device (cf-LVAD) therapy.
METHODSPatientsThis retrospective study was based on a historical cohort of patients older than 18 years who required cf-LVAD therapy (HVAD, HeartWare Inc) between 2009 and 2015 in our institution. The study information was extracted from a local database and was supplemented by medical record review. The research was conducted in accordance with the principles of the Declaration of Helsinki and the the study was approved by the ethics committee of the center.
All patients with concomitant cardiac surgery were excluded from the study. The NRI was determined using the modified formula as NRI = (1.519 × serum albumin [g/L]) + 41.7 × (current body weight [kg]/IBW [kg]). The IBW was estimated using the Lorentz formulae for men, namely, IBW = height (cm) – 100 – (height [cm] – 150)/4, and for women, namely, IBW = height (cm) – 100 – (height [cm] – 150)/2.5.
As described in previous studies,13,15 a value of 1 was assigned to the term (current body weight [kg]/IBW [kg]) when the result was ≥ 1. Serum albumin values and body weight were evaluated at the closest time point before cf-LVAD implantation.
Postoperative Clinical EventsRight-sided HF was defined as proposed by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS): elevated central venous pressure (>16mmHg) with depressed cardiac index (<2 L/min/m2) in the absence of elevated pulmonary wedge pressure > 18mmHg requiring implantation of a right ventricular assist device or prolonged (> 1 week) administration of nitric oxide or inotropic therapy. Respiratory failure was defined as pulmonary insufficiency requiring intubation and ventilation for 96hours or more at any time during the postoperative stay due to a blood oxygen saturation < 96% despite respiratory assistance with a fraction of inspired oxygen ≥ 0.50. Major bleeding was defined as an episode of internal or external bleeding causing 1 or more of the following: death, reoperation, hospitalization, or red blood cell transfusion (the first 7 days after implantation: ≥ 4 packed red blood cells in 24hours; ≥ 7 days after implantation: any transfusion of packed red blood cells). Acute renal failure was defined by a new need for dialysis or an increase in serum creatinine to 3 times higher than its baseline value or to > 5mg/dL. Postoperative infection was defined as any infection proven by microbiological isolation requiring treatment with intravenous antibiotics during the postoperative stay. Vital status at 1 year of the cf-LVAD was determined in all patients. Death from any cause during this period was the main study end point.
Statistical AnalysisIn this work, qualitative variables are reported as frequency and percentage and continuous variables as mean ± standard deviation when normally distributed or as median [interquartile range] when not. For comparison of the initial characteristics between the different NRI categories, the Pearson chi-square test was used for qualitative variables. The ANOVA test with a first-order polynomial contrast was applied for quantitative variables.
A Cox proportional hazards model was applied to explore the relationship between the variables and the end point after cf-LVAD implantation. The variables were consecutively tested in a backward stepwise Cox multiple regression model to determine the independent predictors of death. The variables used to calculate the NRI were not included in the multivariable models. The NRI was analyzed as a continuous variable. Statistical significance was set at a P value of < .05. Only variables with P < .05 in the univariable model were included in the multivariable analysis. Finally, the Kaplan-Meier method was used to generate survival curves during the first year after cf-LVAD implantation according to nutritional risk. The survival curves were assessed with the log-rank test. The level of statistical significance was set at P < .05 for all comparisons. Statistical analysis was performed with SPSS 20.0.
RESULTSNutritional Risk Before Implantation of a Continuous-flow Left Ventricular Assist DeviceFrom January 2009 to December 2015, 279 patients older than 18 years underwent cf-LVAD therapy (HVAD, HeartWare Inc). Before implantation, 15 patients (5.4%) had severe nutritional risk (NRI < 83.5), 60 (21.5%) had moderate nutritional risk (83.5 ≤ NRI < 97.5), and 26 (9.3%) had mild nutritional risk (97.5 ≤ NRI < 100); 178 (63.8%) showed no evidence of malnutrition (NRI ≥ 100). The average preoperative NRI was 105.3 ± 13.6.
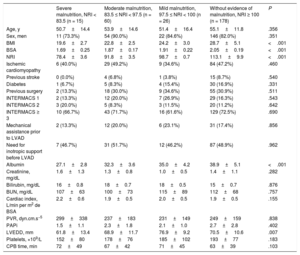
The initial characteristics of the subgroups according to NRI quartile are summarized in Table 1. Patients with a lower preoperative NRI had a lower body mass index (P < .001) and body surface area (P < .001), lower serum albumin (P < .001), and smaller ventricles (P = .007). Although the differences were not statistically significant, there were higher percentages of patients with INTERMACS 1 in the group with mild nutritional risk and of patients with INTERMACS 2 in the group with severe nutritional risk. The group with high nutritional risk also contained fewer patients with a history of cardiac surgery (P = .511). A direct proportional relationship was also observed between the number of platelets and the NRI.
Clinical Characteristics of Study Patients According to Their Nutritional Risk Before LVAD Implantation
| Severe malnutrition, NRI < 83.5 (n = 15) | Moderate malnutrition, 83.5 ≤ NRI < 97.5 (n = 60) | Mild malnutrition, 97.5 ≤ NRI < 100 (n = 26) | Without evidence of malnutrition, NRI ≥ 100 (n = 178) | P | |
|---|---|---|---|---|---|
| Age, y | 50.7±14.4 | 53.9±14.6 | 51.4±16.4 | 55.1±11.8 | .356 |
| Sex, men | 11 (73.3%) | 54 (90.0%) | 22 (84.6%) | 146 (82.0%) | .351 |
| BMI | 19.6±2.7 | 22.8±2.5 | 24.2±3.0 | 28.7±5.1 | <.001 |
| BSA | 1.69±0.25 | 1.87±0.17 | 1.91±0.22 | 2.05±0.19 | <.001 |
| NRI | 78.4±3.6 | 91.8±3.5 | 98.7±0.7 | 113.1±9.9 | <.001 |
| Ischemic cardiomyopathy | 6 (40.0%) | 29 (49.2%) | 9 (34.6%) | 84 (47.2%) | .460 |
| Previous stroke | 0 (0.0%) | 4 (6.8%) | 1 (3.8%) | 15 (8.7%) | .540 |
| Diabetes | 1 (6.7%) | 5 (8.3%) | 4 (15.4%) | 30 (16.9%) | .331 |
| Previous surgery | 2 (13.3%) | 18 (30.0%) | 9 (34.6%) | 55 (30.9%) | .511 |
| INTERMACS 1 | 2 (13.3%) | 12 (20.0%) | 7 (26.9%) | 29 (16.3%) | .543 |
| INTERMACS 2 | 3 (20.0%) | 5 (8.3%) | 3 (11.5%) | 20 (11.2%) | .642 |
| INTERMACS ≥ 3 | 10 (66.7%) | 43 (71.7%) | 16 (61.6%) | 129 (72.5%) | .690 |
| Mechanical assistance prior to LVAD | 2 (13.3%) | 12 (20.0%) | 6 (23.1%) | 31 (17.4%) | .856 |
| Need for inotropic support before LVAD | 7 (46.7%) | 31 (51.7%) | 12 (46.2%) | 87 (48.9%) | .962 |
| Albumin | 27.1±2.8 | 32.3±3.6 | 35.0±4.2 | 38.9±5.1 | <.001 |
| Creatinine, mg/dL | 1.6±1.3 | 1.3±0.8 | 1.0±0.5 | 1.4±1.1 | .282 |
| Bilirubin, mg/dL | 16±0.8 | 18±0.7 | 18±0.5 | 15±0.7 | .876 |
| BUN, mg/dL | 107±63 | 100±73 | 115±89 | 112±68 | .757 |
| Cardiac index, L/min per m2 de BSA | 2.2±0.6 | 1.9±0.5 | 2.0±0.5 | 1.9±0.5 | .155 |
| PVR, dyn.cm.s−5 | 299±338 | 237±183 | 231±149 | 249±159 | .838 |
| PAPi | 1.5±1.1 | 2.3±1.8 | 2.1±1.0 | 2.7±2.8 | .402 |
| LVEDD, mm | 61.8±13.4 | 68.9±11.7 | 76.9±9.2 | 70.5±10.6 | .007 |
| Platelets, ×109/L | 152±80 | 178±76 | 185±102 | 193±77 | .183 |
| CPB time, min | 72±49 | 67±42 | 71±45 | 63±39 | .103 |
BMI, body mass index; BSA, body surface area; BUN, blood urea nitrogen; CPB, cardiopulmonary bypass; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; NRI, nutritional risk index; PAPi, pulmonary artery pulsatility index; PVR, pulmonary vascular resistance.
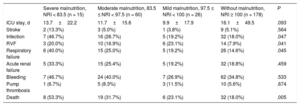
The adverse clinical events occurring during the postoperative period after the cf-LVAD therapy are summarized in Table 2. Patients were divided into 4 subgroups according to their preoperative NRI. The incidences of right ventricular failure (P = .041), respiratory failure (P = .045), and infections (P = .047) were inversely correlated with the respective preoperative NRI values. Although the difference was not significant, patients at risk of malnutrition had a shorter stay in the intensive care unit (P = .093) than those without risk. No differences were observed in the incidences of acute renal failure, stroke, pump thrombosis, or postoperative bleeding.
Clinical Events During Postoperative Stay After Left Ventricular Assist Device Therapy According to Preoperative Nutritional Risk Index
| Severe malnutrition, NRI < 83.5 (n = 15) | Moderate malnutrition, 83.5 ≤ NRI < 97.5 (n = 60) | Mild malnutrition, 97.5 ≤ NRI < 100 (n = 26) | Without malnutrition, NRI ≥ 100 (n = 178) | P | |
|---|---|---|---|---|---|
| ICU stay, d | 13.7±22.2 | 11.7±15.6 | 9.9±17.9 | 16.1±49.5 | .093 |
| Stroke | 2 (13.3%) | 3 (5.0%) | 1 (3.8%) | 9 (5.1%) | .564 |
| Infection | 7 (46.7%) | 16 (26.7%) | 5 (19.2%) | 32 (18.0%) | .047 |
| RVF | 3 (20.0%) | 10 (16.9%) | 6 (23.1%) | 14 (7.9%) | .041 |
| Respiratory failure | 6 (40.0%) | 15 (25.0%) | 5 (19.2%) | 26 (14.6%) | .045 |
| Acute renal failure | 5 (33.3%) | 15 (25.4%) | 5 (19.2%) | 32 (18.8%) | .459 |
| Bleeding | 7 (46.7%) | 24 (40.0%) | 7 (26.9%) | 62 (34.8%) | .533 |
| Pump thrombosis | 1 (6.7%) | 5 (8.3%) | 3 (11.5%) | 10 (5.6%) | .674 |
| Death | 8 (53.3%) | 19 (31.7%) | 6 (23.1%) | 32 (18.0%) | .005 |
ICU, intensive care unit; NRI, nutritional risk index; RVF, right ventricular failure.
Multivariable logistic regression analysis identified preoperative NRI as a significant independent predictor of right ventricular failure after cf-LVAD implantation (adjusted odds ratio [aOR] per 1 unit = 0.963, 95% confidence interval [95%CI], 0.934-0.992, P = .014), respiratory failure (aOR per 1 unit = 0.961, 95%CI, 0.936-0.987, P = .004), and postoperative infection (aOR per 1 unit = 0.968, 95%CI, 0.946-0.991, P = .007) (Table 3).
Independent Predictors of the Incidence of Right Ventricular Failure, Respiratory Failure, and Postoperative Infection and Predictors of Mortality During the First Year After LVAD Implantation: Multivariable Analysis
| OR (95%CI) | P | |
|---|---|---|
| Right ventricular failure | ||
| NRI (per 1 unit) | 0.963 (0.934-0.992) | .014 |
| Previous cardiac surgery | 2.325 (1.065-5.078) | .034 |
| Increased pulmonary resistances before implantation | 2.652 (1.238-5.683) | .012 |
| Bilirubin (each 1 mg/dL) | 1.011 (1.001-1.021) | .042 |
| INTERMACS 1 vs > 2 | 2.270 (1.128-4.569) | .022 |
| INTERMACS 2 vs > 2 | 1.983 (0.993-4.272) | .057 |
| Respiratory failure | ||
| NRI (per 1 unit) | 0.961 (0.936-0.987) | .004 |
| Diabetes mellitus | 1.434 (0.979-2.321) | .053 |
| Previous cardiac surgery | 3.838 (1.852-7.952) | <.001 |
| INTERMACS 1 vs > 2 | 3.831 (1.553-9.453) | .004 |
| INTERMACS 2 vs > 2 | 2.203 (1.004-4.835) | .049 |
| Infection | ||
| NRI (per 1 unit) | 0.968 (0.946-0.991) | .007 |
| INTERMACS 1 vs > 2 | 2.359 (0.095-5.963) | .051 |
| INTERMACS 2 vs > 2 | 2.019 (0.993-4.105) | .052 |
| Mechanical assistance prior to LVAD | 2.112 (1.221-3.896) | .023 |
| Diabetes mellitus | 1.522 (1.020-2.984) | .047 |
| Predictors of mortality during the first year after LVAD implantation | HR (95%CI) | P |
|---|---|---|
| NRI (per 1 unit) | 0.961 (0.941-0.981) | <.001 |
| Previous cardiac surgery | 2.154 (1.295-3.583) | .034 |
| Baseline serum creatinine (each 1 mg/dL) | 1.452 (1.104-3.790) | .021 |
| Year (each 1 y) | 1.029 (0.999-1.060) | .056 |
| INTERMACS 1 vs > 2 | 3.995 (2.253-7.082) | <.001 |
| INTERMACS 2 vs > 2 | 3.230 (1.638-6.370) | .001 |
95%CI, 95% confidence interval; HR, hazard ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; NRI, nutritional risk index; OR, odds ratio; PVR, pulmonary vascular resistance.
Increase in pulmonary resistance before implantation (PVR > 3 WU).
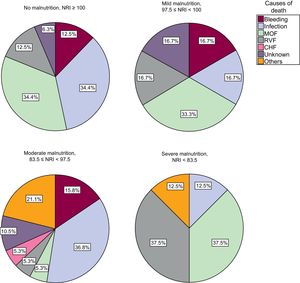
During the first year of follow-up after cf-LVAD therapy, 65 patients died (23.3%). Considering the preoperative nutritional risk (severe, moderate, mild, or absent), the mortality rates of the first year were 53.3%, 31.7%, 23.1%, and 18.0% (P = .005), respectively. The main causes of death during the first year were infection (30.8%), multiorgan failure (26.2%), right ventricular failure (13.2%), and bleeding (12.3%). The distribution of the cause of death according to NRI is shown in Figure 1.
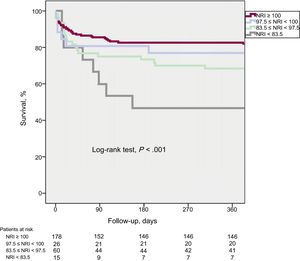
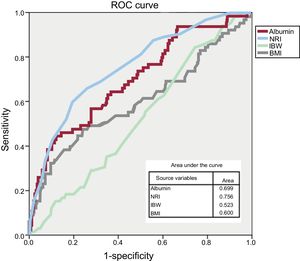
Preoperative NRI was identified in Cox multivariate regression analysis as an independent predictor of lower risk of death from any cause during the first year after cf-LVAD therapy (adjusted hazard ratio per 1 unit = 0.961, 95%CI, 0.941-0.981, P < .001). INTERMACS classification, a history of cardiac surgery, age, and baseline creatinine were also independent predictors of mortality during the first year after implantation (Table 3). Kaplan-Meier survival curves according to NRI subgroup based on malnutrition risk are shown in Figure 2 (log-rank test, P < .001). The ROC curves of the different factors associated with nutrition–NRI, albumin, percentage of ideal weight, and body mass index–are compared in Figure 3; as evident in the figure, the NRI occupies a larger area on the curve.
Our results show that the NRI is a useful prognostic marker for candidates for cf-LVAD therapy. In the present series, 36.2% of the patients were at risk of malnutrition. The largest subgroup comprised patients with moderate nutritional risk (21.5%). There were no significant differences between the subgroups according to their INTERMACS classification and their NRI. However, the NRI was independently associated with the main complications of cf-LVAD therapy: right ventricular failure, infection, respiratory failure, and mortality during follow-up. The association between NRI and mortality was still significant after adjustment for multiple potential confounding factors.
Nutrition is an aspect of growing importance within the pathophysiology of HF. The pathogenesis of malnutrition has been linked to the catabolic state imposed by the disease, by either neurohormonal or immunoinflammatory mechanisms.9 Although nutritional status had already been identified as an independent prognostic factor in various cohorts of patients with HF, the medical literature on candidates for cf-LVAD therapy is scarce.16 Aggarwal et al.16 conducted a nutritional study involving a previously validated questionnaire to evaluate candidates for LVAD therapy and heart transplant. The authors observed higher mortality in patients with worse nutritional status. Malnourished individuals show an increased risk of adverse postoperative events in various surgical settings.17 Malnutrition in patients with a cf-LVAD contributes to a series of postoperative problems, such as infection and limited functional capacity, which can compromise long-term outcomes. In addition, malnutrition is a recognized cause of immunodeficiency, which increases the risk of postoperative infections through various pathophysiological mechanisms.18 The nutritional status of patients after cf-LVAD implantation has been linked to a higher incidence of transmission line infections.19 Malnourished critically-ill patients are also predisposed to respiratory muscle dysfunction, which hinders early weaning from mechanical ventilation.20 This finding was replicated in this series. Complications associated with malnutrition often significantly increase length of postoperative stay, health care costs, and mortality.17,21 However, in the present series, patients at risk of malnutrition showed a tendency for a shorter stay in the intensive care unit (P = .093) than those without this risk. This observation is probably biased by a higher incidence of early mortality among malnourished patients together with fewer patients in the malnutrition groups. In addition, malnourished patients can show platelet a*l*t*erations that affect both platelet number and activity22; hemostasis and platelet function are altered in patients with HF.23 Both factors can influence the NRI, and a directly proportional but nonsignificant relationship was observed between the number of platelets and the NRI in the present series. Equally, no differences were observed in the incidence of postoperative bleeding among the groups. Regardless of the preimplantation number of platelets, the bleeding rate might be influenced by intraoperative transfusion with platelet concentrates, a factor that depends on the institutional protocol.
The NRI is a simple and rapid method to calculate the individual nutritional risk of patients with HF that shows considerable prognostic value. Its standard use in the evaluation of patients for cf-LVAD implantation can provide additional information for patient selection, but can also help to identify those who may require additional nutritional therapy before implantation.24
Barge-Caballero et al.25 recently observed a worse prognosis in malnourished patients who underwent heart transplant. This association is important because a common argument for the implantation of an LVAD as a bridge to transplant is an improvement in patients’ general and nutritional status. However, our data indicate that these patients continue to have worse prognosis after device implantation than those with good nutritional status. Therefore, from our point of view, it seems beneficial to incorporate nutrition into each therapeutic plan for patients with a cf-LVAD. Although malnutrition should not be a contraindication for this type of therapy, its presence in patients with advanced HF should alert physicians to a possible poor outcome. Therefore, the early introduction of nutritional supplementation can improve nutritional status and, subsequently, the general prognosis.26 Finally, to improve the outcomes of patients with cf-LVAD, early implantation in a nutritional stage prior to cardiac cachexia should be a priority.
LimitationsThis study was conducted in a single large tertiary referral center; therefore, the results may not be generalizable. Although retrospective in nature, the study was performed in prospectively compiled data, which involves inherent limitations, such as an inability to adjust for unknown confounding factors and a referral bias. Because the single-center setting of the study weakens the external validity of its results, the conclusions cannot be directly extrapolated to other populations. Although data were collected using the last available blood test before cf-LVAD implantation, the retrospective use of these data cannot exclude the possibility that these values could have varied in the time from the preoperative determination of patients’ albumin and body weight to the implantation.
CONCLUSIONSThe present study confirms the clinical usefulness of the NRI as a screening tool for the nutritional status of patients with advanced HF who are candidates for cf-LVAD therapy. Malnourished patients show higher mortality in the first year after cf-LVAD implantation due to a significant increase in the incidence of postoperative complications, such as infections, respiratory failure, and right ventricular failure. These results demonstrate the need for continued examination of the potential clinical benefit of interventions designed to improve the nutritional status of patients with HF.
FUNDINGThis research did not receive any specific financial support from any funding agency in the public, commercial, or nonprofit sectors.
CONFLICTS OF INTERESTS.V. Rojas and M. Avsar are consultants for Medtronic and Abbott.
- –
Malnutrition is a common comorbidity in patients with HF and is associated with higher mortality.
- –
The NRI is calculated from the serum albumin concentration and the relationship between patients’ actual and ideal weights and has been linked to prognosis in this population.
- –
The NRI may play a similar role in patients with a LVAD, but this specific group has not yet been studied.
- –
The present study confirms the value of this nutritional risk score as an independent predictor of prognosis in patients undergoing LVAD therapy.
- –
Assessment of malnourished patients who are being evaluated for LVAD implantation could identify higher-risk candidates and ensure that they begin a nutritional intervention at an early stage.