The tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/SPAP) ratio is a noninvasive surrogate of right ventricular to pulmonary circulation that has prognostic implications in patients with heart failure (HF) or pulmonary hypertension. Our purpose was to evaluate the prognostic value of the TAPSE/SPAP ratio in patients with cardiac amyloidosis.
MethodsWe used the database of the AMIGAL study, a prospective, observational registry of patients with cardiac amyloidosis recruited in 7 hospitals of the Autonomous Community of Galicia, Spain, from January 1, 2018 to October 31, 2022. We selected patients whose baseline TAPSE/SPAP ratio was calculated with transthoracic echocardiography. Long-term survival and survival free of HF hospitalization were assessed by means of 5 different multivariable Cox regression models. Median follow-up was 680 days.
ResultsWe studied 233 patients with cardiac amyloidosis, among whom 209 (89.7%) had transthyretin type. The baseline TAPSE/SPAP ratio correlated significantly with clinical outcomes. Depending on the multivariable model considered, the adjusted hazard ratios estimated per 0.1mm/mmHg increase of baseline TAPSE/SPAP ratio ranged from 0.76 to 0.84 for all-cause mortality. Similarly, the ratios for all-cause mortality of HF hospitalization ranged from 0.79 to 0.84. The addition of the baseline TAPSE/SPAP ratio to the predictive model of the United Kingdom National Amyloidosis Centre resulted in an increase in Harrell's c-statistic from 0.662 to 0.705 for all-cause mortality and from 0.668 to 0.707 for all-cause mortality or HF hospitalization.
ConclusionsReduced TAPSE/SPAP ratio is an independent adverse prognostic marker in patients with cardiac amyloidosis.
Keywords
Cardiac amyloidosis is a family of infiltrative diseases caused by myocardial deposition of amyloid fibrils, most frequently composed of monoclonal immunoglobulin light chains (AL type) or misfolded transthyretin (ATTR type), either in its variant/hereditary or wild-type/nonhereditary subtypes.1,2
The most frequent clinical phenotype of cardiac amyloidosis is heart failure (HF) with preserved ejection fraction, which may progress to severe restrictive cardiomyopathy.3,4 Pulmonary hypertension can develop as a result of the backward transmission of increased left ventricular filling pressures to the pulmonary circulation, adversely affecting prognosis.5,6 In this clinical scenario, the adequacy of right ventricle adaptation to increased afterload is a known determinant of the severity of symptoms and long-term outcomes.7,8
Invasive cardiac catheterization remains the gold standard to evaluate right ventricle to pulmonary artery coupling.9 However, it also may be reliably assessed by means of a noninvasive surrogate, the ratio between tricuspid annular plane systolic excursion (TAPSE) and systolic pulmonary arterial pressure (SPAP), which can be measured with transthoracic echocardiography.10,11 Previous studies have shown that a reduced TAPSE/SPAP ratio, which is indicative of right ventricle to pulmonary artery uncoupling, is a strong independent predictor of adverse outcomes in patients with primary pulmonary hypertension10,12 or HF with either reduced or preserved ejection fraction.11,13
The prognostic implications of the TAPSE/SPAP ratio in patients with cardiac amyloidosis have not yet been clarified. Understanding the potential role of this parameter could provide further insights into the natural history of the disease, as well as a meaningful and feasible noninvasive prognostic tool than might be used in addition to validated biomarker-based staging systems.14,15 Therefore, in this study, we aimed to describe the distribution of the TAPSE/SPAP ratio and to evaluate its prognostic implications in a multi-institutional cohort of patients with cardiac amyloidosis.
METHODSStudy designClinical data used for the present investigation were extracted from the database of the observational, prospective, multicenter AMIGAL registry, which includes consecutive patients with amyloid cardiomyopathy recruited in 7 hospitals of the public health care system of the Autonomous Community of Galicia (figure 1). Patient enrolment started on January 1, 2018 and is still ongoing. For the present analysis, we selected patients who had been included in the registry from the beginning until October 31, 2022.
According to the study protocol, described elsewhere,16 diagnosis of AL cardiac amyloidosis is established by the detection of light-chain deposits in endomyocardial biopsy specimens alone or in a noncardiac biopsy, together with highly suggestive cardiac imaging findings. ATTR cardiac amyloidosis is defined by a grade ≥ 2 cardiac uptake on 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in patients with no evidence of monoclonal gammopathy in serum and urine immunofixation and serum free light-chain assay. Histological confirmation of transthyretin deposits is required only in the presence of monoclonal gammopathy. A genetic test to reveal pathogenic variants of the transthyretin gene is recommended in patients with ATTR cardiac amyloidosis. The diagnosis of other types of cardiac amyloidosis different to AL and ATTR, eg, Apo-A IV cardiac amyloidosis, requires the demonstration of an abnormal deposition of the causal protein in endomyocardial biopsy specimens by means of mass spectrophotometry.
The study protocol was approved by the Ethics Committee for Clinical Research of Galicia, and written informed consent was obtained from all participants before enrollment. The project conforms to the principles outlined in the Declaration of Helsinki.
EchocardiographyTransthoracic echocardiography was performed by board-certified cardiologists during routine clinical follow-up and according to practice guidelines.17 Data on echocardiographic parameters were collected from echocardiographic reports available in the clinical history of each patient.
Systolic pulmonary artery pressure was calculated as the sum of maximum systolic transtricuspid gradient, measured by means of continuous flow Doppler in the apical 4-chamber view, plus central venous pressure, estimated on the basis of the diameter and respiratory variation of the inferior vena cava in the subxiphoid view.
Specific guidelines for the echocardiographic estimation of central venous pressure17 recommend assigning a value of 3mmHg in cases where the diameter of the inferior vena cava is ≤ 21mm and collapses> 50% with a sniff and assigning a value of 15mmHg in cases where the diameter of the inferior vena cava is> 21mm and collapses <50% with a sniff. In intermediate cases that do not fit this paradigm, the guidelines recommend assigning a value of 8mmHg.
Tricuspid annulus plane systolic excursion was measured by means of the M-mode in the apical 4-chamber view.
Patients included in the registry who had missing data for calculating the TAPSE/SPAP ratio were excluded from the present analysis.
Follow-up and study outcomesPatients were followed up from the day of their inclusion in the registry until November 30, 2022, or until the date of death or heart transplant, whichever occurred first. Major clinical outcomes evaluated in this study were overall survival and survival free of HF hospitalization. In the assessment of the combined endpoint, hospitalization for heart transplant was considered as an equivalent of HF hospitalization.
Causes of death were collected from death certificates or, when available, from autopsy reports. For statistical analysis, unknown causes of death were considered as being of a cardiovascular origin.
Statistical analysesFor statistical comparisons, patients were categorized into 3 groups according to tertiles of the baseline TAPSE/SPAP ratio. Categorical variables are reported as absolute numbers and percentages, while continuous variables are expressed as means±standard deviation or as medians [interquartile range], as appropriate. The Kolmogorov-Smirnov test was used to evaluate the conformity of continuous variables to normal distribution.
Comparison of baseline characteristics, echocardiographic parameters, and laboratory data across TAPSE/SPAP tertiles was performed by using the chi-square test for linear trends for categorical variables and by using analysis of variance for linear trends in the case of continuous ones.
Cumulative survival and cumulative survival free of HF hospitalization are summarized by Kaplan-Meier survival curves and were compared across tertiles of TAPSE/SPAP ratio by means of the log-rank test.
We used multivariable Cox proportional-hazards regression analysis to adjust the effect of confounding bias on the statistical associations observed between baseline TAPSE/SPAP ratio and the risk of all-cause mortality and the combined endpoint of all-cause mortality or HF hospitalization. We rejected the use of backward stepwise models to identify potential confounders to avoid possible loss of information due to limited statistical power. Instead, we designed 5 different Cox regression models in which we entered the clinical, echocardiographic, analytical, treatment or staging variables that we considered as potential confounders, on the basis of previous knowledge and the distribution of this variables across categories of the TAPSE/PSAP ratio. Thus, our primary goal with this analysis was to control potential confounding bias, by calculating estimated hazard ratios adjusted by different combinations of relevant clinical characteristics.
For this purpose, we designed 5 multivariable models that included different combinations of adjusting baseline covariables that were distributed asymmetrically across tertiles of the TAPSE/SPAP ratio and, therefore, were considered as potential confounders. Model 1 (clinical co-variables) included age, gender, type of cardiac amyloidosis (ATTR vs other types), atrial fibrillation, New York Heart Association (NYHA) class, and physical signs of congestion. Model 2 (laboratory covariables) included NT-proBNP, glomerular filtration rate, uric acid, urea, gamma-glutamyl transpherase, alkaline phosphatase, and potassium. Model 3 (echocardiographic variables) included maximum left ventricular wall thickness, left ventricular ejection fraction, moderate or severe mitral regurgitation, and moderate or severe tricuspid regurgitation. Model 4 (treatment variables) included the use of loop diuretics, beta-blockers, mineralocorticoid receptor antagonists, and anticoagulants. Model 5 (disease stages) included cardiac amyloidosis stages, as defined by means of the United Kingdom (UK) National Amyloid Centre (NAC) staging system,15 a validated prognostic model based on serum NT-proBNP concentrations and glomerular filtration rate.
We calculated Harrell's c-statistics to evaluate the predictive ability of the UK NAC staging system15 in our sample, as well as the incremental predictive capacity of adding the TAPSE/SPAP ratio to this biomarker-based model. In addition, we evaluated the association between the TAPSE/SPAP ratio and study outcomes stratified by UK NAC stages. Comparisons of Harrell's c-statistics between 2 different Cox regression models were performed by means of the Comparec-statistical package for R.
A 2-tailed P value <.05 was used to indicate statistical significance. Analyses were performed with SPSS 25 (IBM, United States), STATA 14 (Statacorp, United States) and R 4.3.2 (R Foundation, Austria).
RESULTSPatientsA total of 315 patients with cardiac amyloidosis were included in the registry from January 1, 2018 to October 31, 2022. Among them, 233 (74.0%) had available baseline echocardiographic data for calculating the TAPSE/SPAP ratio, and constituted the population assessed in the present analysis. In 72 out of the 82 (87.8%) patients excluded from the study, lack of data were due to the presence of a trace or no tricuspid regurgitation on baseline echocardiography, and consequently the transtricuspid gradient could not be calculated to estimate SPAP.
Of the final study cohort, 209 (89.7%) patients had ATTR amyloid cardiomyopathy, 23 (9.9%) had AL amyloid cardiomyopathy and 1 (0.4%) had Apo-A IV amyloid cardiomyopathy. Among ATTR cases, 176 (84.2%) were confirmed wild-type, 6 (2.9%) were confirmed variant cases, and 27 (12.9%) were indeterminate, as these patients were not genotyped.
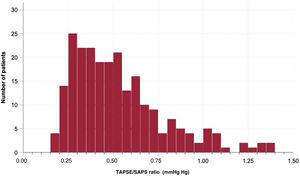
Figure 2 shows the distribution of the baseline TAPSE/SPAP ratio in the study population. The median value of this parameter was 0.48mm/mmHg, and the interquartile range was 0.33-0.65mm/mmHg. The first (lowest), second (intermediate) and third (highest) tertiles of the TAPSE/SPAP ratio were <0.38mm/mmHg, 0.38 to 0.58mm/mmHg and> 0.58mm/mmHg, respectively.
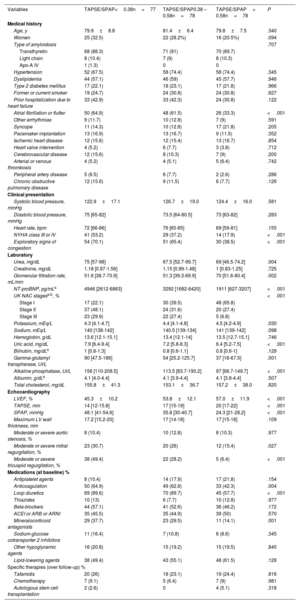
Baseline clinical characteristicsThe patients’ baseline clinical, laboratory and echocardiographic characteristics are shown in table 1 and classified according to tertiles of the TAPSE/SPAP ratio.
Baseline characteristics of study patients according to tertiles of the TAPSE/SPAP ratio
| Variables | TAPSE/SPAP<0.38n=77 | TAPSE/SPAP0.38 – 0.58n=78 | TAPSE/SPAP> 0.58n=78 | P |
|---|---|---|---|---|
| Medical history | ||||
| Age, y | 79.9±8.8 | 81.4±6.4 | 79.8±7.5 | .340 |
| Women | 25 (32.5) | 22 (28.2%) | 16 (20.5%) | .094 |
| Type of amyloidosis | .707 | |||
| Transthyretin | 68 (88.3) | 71 (91) | 70 (89.7) | |
| Light chain | 8 (10.4) | 7 (9) | 8 (10.3) | |
| Apo-A IV | 1 (1.3) | 0 | 0 | |
| Hypertension | 52 (67.5) | 58 (74.4) | 58 (74.4) | .345 |
| Dyslipidemia | 44 (57.1) | 46 (59) | 45 (57.7) | .946 |
| Type 2 diabetes mellitus | 17 (22.1) | 18 (23.1) | 17 (21.8) | .966 |
| Former or current smoker | 19 (24.7) | 24 (30.8) | 24 (30.8) | .627 |
| Prior hospitalization due to heart failure | 33 (42.9) | 33 (42.3) | 24 (30.8) | .122 |
| Atrial fibrillation or flutter | 50 (64.9) | 48 (61.5) | 26 (33.3) | <.001 |
| Other arrhythmias | 9 (11.7) | 10 (12.8) | 7 (9) | .591 |
| Syncope | 11 (14.3) | 10 (12.8) | 17 (21.8) | .205 |
| Pacemaker implantation | 13 (16.9) | 13 (16.7) | 9 (11.5) | .352 |
| Ischemic heart disease | 12 (15.6) | 12 (15.4) | 13 (16.7) | .854 |
| Heart valve intervention | 4 (5.2) | 6 (7.7) | 3 (3.8) | .712 |
| Cerebrovascular disease | 12 (15.6) | 8 (10.3) | 7 (9) | .200 |
| Arterial or venous thrombosis | 4 (5.2) | 4 (5.1) | 5 (6.4) | .742 |
| Peripheral artery disease | 5 (6.5) | 6 (7.7) | 2 (2.6) | .286 |
| Chronic obstructive pulmonary disease | 12 (15.6) | 9 (11.5) | 6 (7.7) | .126 |
| Clinical presentation | ||||
| Systolic blood pressure, mmHg | 122.9±17.1 | 126.7±19.0 | 124.4±16.0 | .581 |
| Diastolic blood pressure, mmHg | 75 [65-82] | 73.5 [64-80.5] | 73 [63-82] | .283 |
| Heart rate, bpm | 72 [66-86] | 76 [65-85] | 69 [59-81] | .155 |
| NYHA class III or IV | 41 (53.2) | 29 (37.2) | 14 (17.9) | <.001 |
| Exploratory signs of congestion | 54 (70.1) | 51 (65.4) | 30 (38.5) | <.001 |
| Laboratory | ||||
| Urea, mg/dL | 75 [57-98] | 67.5 [52.7-99.7] | 69 [46.5-74.2] | .004 |
| Creatinine, mg/dL | 1.18 [0.97-1.56] | 1.15 [0.99-1.48] | 1 [0.83-1.25] | .725 |
| Glomerular filtration rate, mL/min | 51.6 [38.7-70.9] | 51.3 [39.3-69.9] | 70 [51.6-80.4] | .002 |
| NT-proBNP, pg/mLa | 4946 [2612-6863] | 3292 [1682-6420] | 1911 [627-3207] | <.001 |
| UK NAC stagesa,b, % | <.001 | |||
| Stage I | 17 (22.1) | 30 (39.5) | 48 (65.8) | |
| Stage II | 37 (48.1) | 24 (31.6) | 20 (27.4) | |
| Stage III | 23 (29.9) | 22 (27.4) | 5 (6.8) | |
| Potassium, mEq/L | 4.3 [4.1-4.7] | 4.4 [4.1-4.8] | 4.5 [4.2-4.9] | .030 |
| Sodium, mEq/L | 140 [138-142] | 140.5 [139-134] | 141 [139-142] | .098 |
| Hemoglobin, g/dL | 13.6 [12.1-15.1] | 13.4 [12.1-14] | 13.5 [12.7-15.1] | .746 |
| Uric acid, mg/dL | 7.9 [6.4-9.4] | 7.2 [5.8-8.3] | 6.4 [5.2-7.5] | <.001 |
| Bilirubin, mg/dLa | 1 [0.8-1.3] | 0.8 [0.6-1.1] | 0.8 [0.6-1] | .129 |
| Gamma-glutamyl transpherase, UI/L | 90 [47.5-186] | 54 [25.2-125.7] | 37 [18-67.5] | .001 |
| Alkaline phosphatase, UI/L | 156 [110-208.5] | 113.5 [83.7-190.2] | 97 [68.7-149.7] | <.001 |
| Albumin, g/dLa | 4.1 [4.0-4.4] | 4.1 [3.9-4.4] | 4.1 [3.8-4.4] | .507 |
| Total cholesterol, mg/dL | 155.8±41.3 | 153.1±36.7 | 157.2±38.0 | .820 |
| Echocardiography | ||||
| LVEF, % | 45.3±10.2 | 53.8±12.1 | 57.0±11.9 | <.001 |
| TAPSE, mm | 14 [12-15.8] | 17 [15-19] | 20 [17-22] | <.001 |
| SPAP, mmHg | 48.1 [41-54.6] | 35.8 [30-40.7] | 24.3 [21-28.2] | <.001 |
| Maximum LV wall thickness, mm | 17.2 [15.2-20] | 17 [14-18] | 17 [15-18] | .109 |
| Moderate or severe aortic stenosis, % | 8 (10.4) | 10 (12.8) | 8 (10.3) | .977 |
| Moderate or severe mitral regurgitation, % | 23 (30.7) | 20 (26) | 12 (15.4) | .027 |
| Moderate or severe tricuspid regurgitation, % | 38 (49.4) | 22 (28.2) | 5 (6.4) | <.001 |
| Medications (at baseline) % | ||||
| Antiplatelet agents | 8 (10.4) | 14 (17.9) | 17 (21.8) | .154 |
| Anticoagulation | 50 (64.9) | 49 (62.8) | 33 (42.3) | .004 |
| Loop diuretics | 69 (89.6) | 70 (89.7) | 45 (57.7) | <.001 |
| Thiazides | 10 (13) | 6 (7.7) | 10 (12.8) | .977 |
| Beta-blockers | 44 (57.1) | 41 (52.6) | 36 (46.2) | .172 |
| ACEI or ARB or ARNI | 35 (45.5) | 35 (44.9) | 39 (50) | .570 |
| Mineralocorticoid antagonists | 29 (37.7) | 23 (29.5) | 11 (14.1) | .001 |
| Sodium-glucose cotransporter 2 inhibitors | 11 (16.4) | 7 (10.8) | 6 (8.6) | .345 |
| Other hypoglycemic agents | 16 (20.8) | 15 (19.2) | 15 (19.5) | .840 |
| Lipid-lowering agents | 38 (49.4) | 43 (55.1) | 48 (61.5) | .128 |
| Specific therapies (over follow-up) % | ||||
| Tafamidis | 20 (26) | 18 (23.1) | 19 (24.4) | .816 |
| Chemotherapy | 7 (9.1) | 5 (6.4) | 7 (9) | .981 |
| Autologous stem cell transplantation | 2 (2.6) | 0 | 4 (5.1) | .318 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; UK NAC, United Kingdom National Amyloidosis Centre.
Categorical variables are presented as number of patients (proportion). Kolmogorov-Smirnov's test was used to evaluate the adequacy of continuous variables to normal distribution. Age, systolic blood pressure, total serum cholesterol and LVEF were normally distributed variables and are therefore expressed as mean±standard deviation. All other continuous variables showed nonnormal distributions and are they are expressed as median [interquartile range].
Stage I: NT-proBNP ≤ 3000 pg/mL and glomerular filtration rate ≥ 45mL/min. Stage III: NT-proBNP> 3000 pg/mL and glomerular filtration rate <45mL/min. Stage II=patients not classified as stage I or stage III.15
There were no significant differences across tertiles of the TAPSE/SPAP ratio in demographic characteristics. However, a lower TAPSE/SPAP ratio was associated with an increased prevalence of atrial fibrillation, NYHA class III or IV, and physical signs of congestion.
The TAPSE/SPAP ratio was inversely associated with serum levels of NT-proBNP, uric acid, alkaline phosphatase, and gamma-glutamyl transpherase; it was also directly associated with glomerular filtration rate. Patients with a TAPSE/PSAP ratio within the highest tertile were more frequently in UK NAC stage I.
On echocardiography, patients with a lower TAPSE/SPAP ratio showed a higher prevalence of moderate or severe tricuspid regurgitation, as well as lower left ventricular ejection fraction.
Prescription of anticoagulants, diuretics and mineralocorticoid antagonists at baseline was significantly more frequent among patients with lower a TAPSE/SPAP ratio, with no differences among study subgroups regarding the prescription of other pharmacological agents.
The proportion of patients who received specific disease modifying therapies such as tafamidis (for ATTR), chemotherapy or autologous stem cell transplantation (for AL) was similar across tertiles of baseline TAPSE/SPAP ratio.
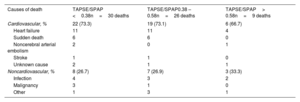
Association between TAPSE/SPAP ratio and clinical outcomesPatients were followed up for a median of 680 [371-1234] days. During this time, 65 (27.9%) patients died and 7 (3.0%) underwent heart transplantation. In addition, 68 (29.2%) patients required at least 1 hospital admission due to HF during follow-up. Cardiovascular causes accounted for 47 out of 65 (72.3%) deaths registered in the study. Table 2 details specific causes of death in the study population, according to tertiles of the baseline TAPSE/SPAP ratio.
Causes of death in the study population according to tertiles of TAPSE/SPAP ratio
| Causes of death | TAPSE/SPAP <0.38n=30 deaths | TAPSE/SPAP0.38 – 0.58n=26 deaths | TAPSE/SPAP> 0.58n=9 deaths |
|---|---|---|---|
| Cardiovascular, % | 22 (73.3) | 19 (73.1) | 6 (66.7) |
| Heart failure | 11 | 11 | 4 |
| Sudden death | 6 | 6 | 0 |
| Noncerebral arterial embolism | 2 | 0 | 1 |
| Stroke | 1 | 1 | 0 |
| Unknown cause | 2 | 1 | 1 |
| Noncardiovascular, % | 8 (26.7) | 7 (26.9) | 3 (33.3) |
| Infection | 4 | 3 | 2 |
| Malignancy | 3 | 1 | 0 |
| Other | 1 | 3 | 1 |
SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annulus plane systolic excursion.
The data are presented as absolute numbers or No. (%).
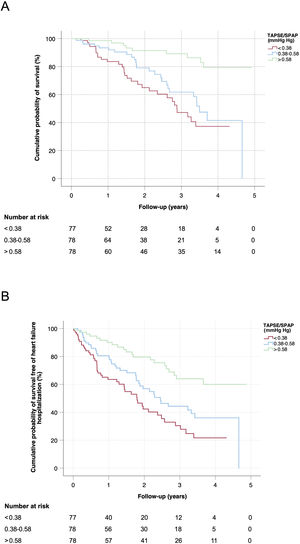
Figure 3 show the Kaplan-Meier estimates for the cumulative probability of survival (A) and the cumulative probability of survival free of HF hospitalization (B) in the study population, stratified according to tertiles of baseline TAPSE/SPAP ratio. In both cases, the log-rank test for linear trends showed a statistically significant association between a lower TAPSE/SPAP ratio and an increased risk of the adverse clinical outcome (P <.001 for both comparisons). The median survival of patients with a baseline TAPSE/SPAP ratio within the first (< 0.38mm/mmHg), second (0.38-0.58mm/mmHg) and third (> 0.58mm/mmHg) tertiles were 2.8, 3.5 and 4.6 years, respectively.
Kaplan-Meier estimates of the overall cumulative probability of survival (A) and the cumulative probability of survival free of heart failure hospitalization (B) in patients with cardiac amyloidosis, stratified by tertiles of baseline TAPSE/SPAP ratio. SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annulus plane systolic excursion.
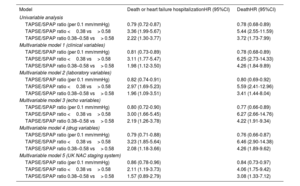
Table 3 summarizes the results of univariate and multivariate Cox regression models constructed to evaluate the association between the baseline TAPSE/SPAP ratio and study outcomes and, thereafter, to adjust potential confounding bias that might affect it. Individual univariate and multivariate coefficients of all explored covariables are presented in tables 1 and 2 of the supplementary data.
Association between baseline the TAPSE/SPAP ratio and long-term clinical outcomes: univariate and multivariate Cox regression analyses
| Model | Death or heart failure hospitalizationHR (95%CI) | DeathHR (95%CI) |
|---|---|---|
| Univariable analysis | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.79 (0.72-0.87) | 0.78 (0.68-0.89) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 3.36 (1.99-5.67) | 5.44 (2.55-11.59) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 2.22 (1.30-3.77) | 3.72 (1.73-7.99) |
| Multivariable model 1 (clinical variables) | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.81 (0.73-0.89) | 0.78 (0.68-0.89) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 3.11 (1.77-5.47) | 6.25 (2.73-14.33) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 1.98 (1.12-3.50) | 4.26 (1.84-9.89) |
| Multivariable model 2 (laboratory variables) | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.82 (0.74-0.91) | 0.80 (0.69-0.92) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 2.97 (1.69-5.23) | 5.59 (2.41-12.96) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 1.96 (1.09-3.51) | 3.41 (1.44-8.04) |
| Multivariable model 3 (echo variables) | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.80 (0.72-0.90) | 0.77 (0.66-0.89) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 3.00 (1.66-5.45) | 6.27 (2.66-14.76) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 2.19 (1.26-3.78) | 4.22 (1.91-9.34) |
| Multivariable model 4 (drug variables) | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.79 (0.71-0.88) | 0.76 (0.66-0.87) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 3.23 (1.85-5.64) | 6.46 (2.90-14.38) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 2.08 (1.18-3.66) | 4.26 (1.89-9.62) |
| Multivariable model 5 (UK NAC staging system) | ||
| TAPSE/SPAP ratio (per 0.1 mm/mmHg) | 0.86 (0.78-0.96) | 0.84 (0.73-0.97) |
| TAPSE/SPAP ratio <0.38 vs> 0.58 | 2.11 (1.19-3.73) | 4.06 (1.75-9.42) |
| TAPSE/SPAP ratio 0.38–0.58 vs> 0.58 | 1.57 (0.89-2.79) | 3.08 (1.33-7.12) |
Adjusting covariables included in multivariable models: Model 1 (baseline clinical variables): age, sex, NYHA class (I to IV), type of cardiac amyloidosis (transthyretin vs other), atrial fibrillation or flutter, physical signs of congestion. Model 2 (baseline laboratory variables): NT-proBNP, glomerular filtration rate, uric acid, urea, gamma-glutamyl transpherase, alkaline phosphatase, potassium. Model 3 (baseline echocardiographic variables): maximum left ventricular wall thickness, left ventricular ejection fraction, moderate or severe mitral regurgitation, moderate or severe tricuspid regurgitation. Model 4 (baseline drug variables): loop diuretic, mineralocorticoid receptor antagonists, anticoagulation, beta-blockers. Model 5 (baseline UK NAC staging system): cardiac amyloidosis stages (I to III). 95%CI, 95% confidence interval; HR, hazard ratio; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annulus plane systolic excursion; UK NAC, United Kingdom National Amyloidosis Centre.
Univariate Cox regression showed a statistically significant association between the baseline TAPSE/SPAP ratio and all-cause mortality (hazard ratio [HR] per 0.1mm/mmHg,0.78; 95% confidence interval [95%CI], 0.68-0.89) and the combined endpoint of all-cause death or HF hospitalization (HR per 0.1mm/mmHg,0.79; 95%CI, 0.72–0.87). Estimated HR for all-cause death were 5.44 (95%CI, 2.55-11.59) for patients who showed a baseline TAPSE/SPAP ratio within the first vs the third tertiles and 3.72 (95%CI, 1.73-7.99) for patients who showed a baseline TAPSE/SPAP ratio within the second vs the third tertiles. Estimated HR for all-cause mortality or HF hospitalization were 3.36 (95%CI, 1.99-5.67) for patients who showed a baseline TAPSE/SPAP ratio within the first vs the third tertiles and 2.22 (95%CI, 1.30-3.77) for patients who showed a baseline TAPSE/SPAP ratio within the second vs the highest tertiles.
The association between baseline TAPSE/SPAP ratio and study outcomes remained as independent and statistically significant in all the 5 adjusted multivariable Cox regression models constructed in this study (table 3). Adjusted HR estimated per 0.1mm/mmHg increase of baseline TAPSE/SPAP ratio ranged from 0.76 to 0.84 for all-cause mortality and from 0.79 to 0.86 for the combined endpoint all-cause death or HF hospitalization. Adjusted HR estimated for patients with baseline TAPSE/SPAP ratio within the first vs the third tertiles ranged from 4.06 to 6.46 for all-cause mortality and from 2.11 to 3.22 for the combined endpoint all-cause death or HF hospitalization. Adjusted HR estimated for patients with baseline TAPSE/SPAP ratio within the second vs the third tertiles varied between 3.08 and 4.26 for all-cause mortality and between 1.57 and 2.19 for the combined endpoint all-cause death or HF hospitalization.
TAPSE/SPAP ratio and cardiac amyloidosis stagesIn the study sample, higher UK NAC stages at baseline were associated with significantly lower cumulative survival and significantly lower cumulative survival free of HF hospitalization (figure 1 of the supplementary data). Increased risk of both adverse outcomes was similar for patients in stage III vs stage I (HR for all-cause death or HF hospitalization,3.32; 95%CI, 1.92-5.75; HR for all-cause death,2.90; 95%CI, 1.41-5.99) and for patients in stage II vs stage I (HR for all-cause death or HF hospitalization,3.90; 95%CI, 2.37-6.42; HR for all-cause death,4.14; 95%CI, 2.16-7.91), with no statistically significant differences between patients at stage III vs stage II (HR for all-cause death or HF hospitalization,0.85; 95%CI, 0.54-1.35; HR for all-cause death,0.70; 95%CI, 0.39-1.27).
Multivariate Cox regression model 5 included baseline UK NAC stages together with baseline TAPSE/SPAP ratio (table 3). According to this model, baseline TAPSE/SPAP ratio was independently associated with the combined endpoint of all-cause death or HF hospitalization (HR per 0.1mm/mmHg=0.86; 95%CI, 0.78-0.96) and all-cause mortality (HR per 0.1mm/Hg=0.84; 95%CI, 0.73-0.97). However, if TAPSE was included in the multivariable model instead of the TAPSE/SPAP ratio, the associations were no longer statistically significant, either with all-cause death or HF hospitalization (HR for 1mm increase of TAPSE=0.96; 95%CI, 0.91-1.02) or for all-cause mortality (HR for 1mm increase of TAPSE,0.95; 95%CI, 0.89-1.02).
UK NAC staging system showed moderate predictive ability both for the combined event of all-cause death or HF hospitalization (Harrell's c-statistic=0.668) and for all-cause mortality (Harrell's c-statistic=0.662). The addition of TAPSE/SPAP ratio to the predictive model based on UK NAC stages resulted in a moderate increase of its predictive capacity, both for the combined event of all-cause death or HF hospitalization (Harrell's c-statistic=0.707; P for comparison=.019) and for all-cause mortality (Harrell's c-statistic=0.705; P for comparison=.065). The addition of TAPSE to the predictive model based on UK NAC stages instead of TAPSE/SPAP ratio resulted in a lower increase of predictive capacity (Harrell's c-statistic for all-cause death or HF hospitalization=0.695; P for comparison=.089; Harrell's c-statistic for all-cause death=0.693; P for comparison=.078).
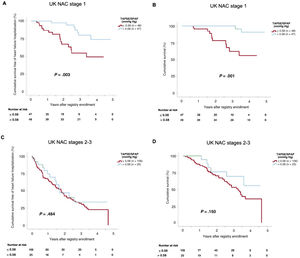
Figure 4 shows the Kaplan-Meier curves of cumulative survival and cumulative survival free of HF hospitalization in patients with baseline TAPSE/SPAP ratio ≤ 0.58 (first and second tertiles) vs> 0.58 (first tertile), categorized according to baseline UK NAC stages. In the subgroup of patients in UK NAC stage I, reduced baseline TAPSE/SPAP ratio (≤ 0.58) was associated with significantly lower survival free of HF hospitalization (P log-rank=.003, figure 4A) and overall survival (P log-rank=.001, figure 4B). However, in the subgroup of patients in UK NAC stages II or III, no statistically significant difference with regard to either adverse outcome were observed according to baseline TAPSE/SPAP ratio (P log-rank for all-cause death or HF hospitalization=.484, figure 4C; P log-rank for all-cause mortality=.150, figure 4D).
Kaplan-Meier estimates of the cumulative survival free of heart failure hospitalization (A,C) and the cumulative survival (B,D) according to United Kingdom National Amyloidosis Centre stages. SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; UK NAC, United Kingdom National Amyloidosis Centre.
In the specific subgroup of patients with ATTR cardiac amyloidosis, estimated Harrell's c-statistics of the UK NAC staging system were 0.652 for the combined endpoint of all-cause mortality or HF hospitalization and 0.640 for all-cause mortality and increased respectively to 0.692 (P for comparison=.030) and 0.686 (P for comparison=.063) when baseline TAPSE/SPAP were added to the predictive model.
DISCUSSIONIn this study, we present the results of a subanalysis of a multicenter registry of patients with amyloid cardiomyopathy that provides further evidence that the TAPSE/SPAP ratio, a noninvasive echocardiographic measurement of right ventricle to pulmonary artery coupling, is a strong independent prognostic marker in this population.
In our study, the presence of severely reduced TAPSE/SPAP ratio at baseline (< 0.38mm/mmHg) was associated with a ∼4 to ∼6-fold increase of the risk of death in patients with cardiac amyloidosis, depending on the multivariable model considered for adjusting residual confounding bias. Notably, even the presence of a mild reduction in this index (< 0.58mm/mmHg) was associated with ∼3 to ∼4-fold increased mortality. Impairment of right ventricle to pulmonary artery coupling was associated with more advanced heart disease, a higher prevalence of atrial fibrillation, worse NYHA class, more frequent signs of congestion, lower left ventricular ejection fraction, higher serum levels of cardiac biomarkers, worse renal function, and an increased incidence of HF hospitalization.
Right ventricular dysfunction is a major determinant of prognosis in patients with HF, either with reduced or preserved ejection fraction.7,18,19 Among the various parameters proposed as surrogates of right ventricular contractility, the assessment of right ventricle to pulmonary artery coupling has received increasing attention in recent years, as it better describes the entire cardiopulmonary unit, addressing the extreme sensitivity of the right ventricular structure to pressure load. The most accurate measurement of right ventricle to pulmonary artery coupling is the ratio between the elastance of the right ventricle and the pulmonary artery, a parameter that can be obtained through invasive cardiac catheterization; 20 a cutoff of 1.5 to 2 represents a good balance between mechanical work and oxygen consumption.21 In patients with pulmonary hypertension, the progressive increase in vascular resistance triggers a maladaptive remodelling of the right ventricle characterized by eccentric hypertrophy and impaired systolic and diastolic function, which leads to a progressive decline in the right ventricle to pulmonary artery elastance ratio up to values <0.8, which define the presence of right ventricle to pulmonary artery uncoupling.22,23
The ratio between TAPSE and SPAP was first validated as a noninvasive marker of right ventricle to pulmonary artery coupling by Guazzi et al.11 in a 2-center prospective study in patients with HF and preserved left ventricular ejection fraction (LVEF). These authors found a good correlation between the TAPSE/SPAP ratio, measured by means of transthoracic echocardiography, and the invasively determined right ventricle to pulmonary artery elastance ratio. In this earlier study,11 a cutoff of TAPSE/SPAP <0.36mm/mmHg was identified as an independent predictor of impaired outcomes. In another study that addressed a broader spectrum of patients with HF irrespective of LVEF, this Italian group found that the TAPSE/SPAP ratio was a more accurate determinant of adverse clinical outcomes than either TAPSE or SPAP alone.13 Later studies have confirmed the predictive role of the TAPSE/SPAP ratio in patients with HF,24 valvular heart disease,25,26 and primary pulmonary hypertension.10
During the last few years, there has been strong interest in providing useful tools to assess the prognosis of patients diagnosed with cardiac amyloidosis and to evaluate progression of the disease. NAC staging system based on NT-proBNP and glomerular filtration rate is widely used and provides good discrimination ability.15 Imaging parameters have also been shown to be independent predictors of mortality; in particular, an article published by Knight et al.27 showed a strong association between TAPSE and prognosis in both AL and ATTR patients. In addition, a recent work by the same group found that progression of mitral regurgitation was the only echocardiographic predictor of mortality during follow-up.28
The rationale for evaluating the potential prognostic value of the TAPSE/SPAP ratio in patients with cardiac amyloidosis is that this disease is a well-known model of restrictive cardiomyopathy, whose typical course involves a clinical picture of HF and normal or mildly reduced ejection fraction, a progressive increase in pulmonary artery pressure and vascular resistance, and subsequent right ventricular dysfunction. Therefore, our results make biological sense and are consistent with previous studies, as they support the use of the TAPSE/SPAP ratio as a surrogate marker of right ventricle to pulmonary artery coupling with prognostic value in patients with cardiac amyloidosis. Of note, the echocardiographic assessment of the TAPSE/SPAP ratio has the advantage of being noninvasive, cost-effective, and easy to perform and monitor over time. Our study suggests that the prognostic value of the TAPSE/SPAP ratio is additive to the that of the UK NAC biomarker-based prognostic model and might be superior to the addition of TAPSE alone.
Of note, in our study, even mild reductions in the TAPSE/SPAP ratio were associated with a significant impairment of cardiovascular outcomes of patients with cardiac amyloidosis. In our opinion, this could be explained by the fact that cardiac amyloidosis is an infiltrative disease in which right ventricular contractility can be directly affected by the deposition of amyloid fibrils in the extracellular space of the right ventricular myocardium. Indeed, our data suggest that the prognostic value of the TAPSE/SPAP ratio is especially useful in patients in UK NAC stage I, that is, in those with less advanced disease. Therefore, the systematic evaluation of the TAPSE/SPAP ratio in patients with cardiac amyloidosis might help clinicians to make an early identification of those at higher risk of disease progression, who may require closer follow-up, as well as those whose disease courts could be modified by specific drugs29,30 or heart transplantation.31 Other authors have previously highlighted the important role of right ventricular function in patients with cardiac amyloidosis.32
Strengths and limitationsThe major strengths of this study are its multi-institutional nature, as well as the prospective inclusion of a substantial number of patients with close clinical follow-up and the collection of comprehensive clinical information. Its major limitations are the observational nature of the study and the absence of external monitoring, which may have led to potential information, selection, and confounding biases. Furthermore, our analysis is based on data previously collected from a general registry of patients with amyloid cardiomyopathy, which was not specifically designed for the purpose of this study. Some prognostic variables of interest, such as serum troponin, were not available for this analysis. Echocardiographic studies were performed locally during routine clinical follow-up by different operators, which may have led to some heterogeneity in the measurement of functional parameters. Finally, data from cardiac catheterization were not available and consequently we could not validate our findings invasively.
CONCLUSIONSThis study supports the prognostic value of the TAPSE/SPAP ratio as a noninvasive, easy to obtain, prognostic marker in patients with cardiac amyloidosis, which may be additive to standard predictive systems based on cardiac biomarkers. Our results highlight the important role of right ventricular to pulmonary afterload coupling in this population.
- –
The TAPSE/SPAP ratio is a noninvasive, echocardiography-derived, surrogate indicator of right ventricle to pulmonary artery coupling.
- –
A reduced TAPSE/SPAP ratio has been associated with adverse prognosis in patients with heart failure independently of LVEF and in patients with primary pulmonary hypertension.
- –
Based on a prospective multicenter registry, our study supports the prognostic usefulness of the TAPSE/SPAP ratio in patients with cardiac amyloidosis.
This study was funded by means of a competitive research grant from the Sociedad Gallega de Cardiología (SOGACAR) and an independent research grant from Pfizer (ID number 54963821).
ETHICAL CONSIDERATIONSThe study protocol was approved by the Committee of Ethics in Clinical Research of the Autonomous Community of Galicia. Written informed consent was obtained from all participants before enrolment. Possible sex/gender biases were not taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence has been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSM. Maccallini: study design, data curation, drafting of the manuscript, study coordination, patient recruitment. G. Barge-Caballero: study design, data curation, drafting of the manuscript, funding acquisition, study coordination, patient recruitment. E. Barge-Caballero: study design, data curation, conceptualization, statistical analysis, drafting of the manuscript, patient recruitment. A. Bouzas-Mosquera: statistical analysis, manuscript reviewing and editing. M.G. Crespo-Leiro: supervision, data curation, patient recruitment, acquisition of funding, visualization, manuscript reviewing and editing. All other authors participated in patient recruitment, data curation, manuscript reviewing and editing.
CONFLICTS OF INTERESTAmong the investigators, 6 (G. Barge-Caballero, E. Barge-Caballero, I. Gómez-Otero, A. Varela-Román, A. Bouzas-Mosquera, and M.G. Crespo-Leiro.) are members of the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) of the Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science and Innovation, Spanish Government. G. Barge-Caballero has received travel grants, speaker fees and a research grant from Pfizer (not related to the present study). E. Barge-Caballero has received speaker fees from Pfizer. M. Crespo-Leiro has received travel grants from Pfizer. R. Bilbao-Quesada has received consultant fees from Pfizer. The remaining authors have nothing to disclose.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2024.01.001.