Keywords
INTRODUCTION
Acute aortic syndrome (AAS) is defined as an acute process in the aortic wall involving a weakening of the medial layer with the risk of aortic rupture and other complications. It consists of three disease entities: aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer.1 It has an incidence of around 30 cases per million per year, of which 80% are aortic dissections, 15% intramural hematomas, and 5% penetrating atherosclerotic ulcers.2,3 The ascending aorta is affected in 60% of cases (type A) and unaffected in 40% (type B). It mainly affects men (70%), with a mean age of 60 years (International Registry of Acute Aortic Dissection [IRAD]).4 In the last decade, progress in imaging techniques has significantly improved diagnosis of AAS and has contributed fundamental information to understanding the evolution of the disease. On the other hand, the development of new surgical techniques and the introduction of endovascular treatment have modified the therapeutic strategy and, probably, the prognosis.
DIAGNOSIS
Clinical Presentation and Exploratory Data
Early diagnosis of this disease is vital for improving prognosis, since mortality is 20% during the first 24 h in its natural evolution and 62% at 7 days.5 It is important to identify the signs and symptoms of the disease to obtain an early diagnosis. Pain is present in 96% of AAS. This pain has several typical characteristics4: a) onset is abrupt and rapidly reaches its maximum intensity; b) it is frequently sharp and located in the mid-chest region when the ascending aorta is involved, or in the interscapular region when the descending aorta is affected; and c) it can be migratory in 25% of cases, or can spread to the jaws, epigastrium, lumbar region, or lower limbs. On the other hand, onset with syncope is relatively frequent (15%).6 Other diseases are initially suspected in more than 30% of the patients finally diagnosed with AAS, such as acute coronary syndrome, pericarditis, pulmonary embolism, osteoarticular disease of the spine, or even cholecystitis. One of the most serious diagnostic errors is to confuse aortic dissection with myocardial infarction, especially if thrombolytic treatment is prescribed. This error can occur if it is not taken into account that the electrocardiogram shows myocardial necrosis patterns in 10% of the patients and signs of ischemia in 15%.4 On the other hand, although it has been traditionally considered that the chest radiograph is always abnormal, recent series show that it can be strictly normal in up to 20% of the patients and there may be mediastinal widening in 60%.4
Clinical background is important when AAS is suspected, since 75% of the patients are hypertensive, 20% have been previously diagnosed with another aortic disease and increasingly more cases are reported associated with aortic valvular heart disease or secondary to iatrogenesis.4 On the other hand, some genetic diseases, such as Marfan syndrome or Ehlers-Danlos syndrome, should lead us to suspect AAS. Intramural hematoma affects patients with more atherosclerotic risk factors and advanced age than aortic dissection and is located in 70% of patients in the descending aorta.7
Physical exploration can clearly reveal severe hypotension or shock in 15% of the patients, aortic regurgitation murmur in 40% of type A dissections and pulse deficit in 20% of AAS.8
Imaging Techniques
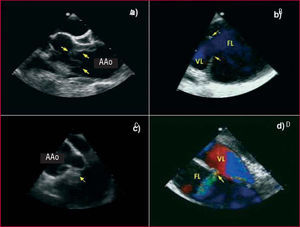
The role of imaging techniques has substantially changed in recent years. Up to 15 years ago it was usual to carry out diagnostic aortography. It was later shown that this technique led to a 20% rate of false negative diagnoses, especially in thrombosed-type A dissections and intramural hematoma. A recently published metaanalysis9 showed that diagnostic accuracy is practically the same (95%-100%) for computerized tomography (CT), transesophageal echocardiography (TEE), and magnetic resonance imaging (MRI). Most shortcomings are due to interpretation errors by the user than to the technique itself. In current series, CT is the most frequently used imaging technique (70%),10 mainly due to its wide availability, accuracy, and speed.11 Its main drawback is the presence of kidney failure. Transesophageal echocardiography has advantages over CT in that it can more frequently identify the location and size of the entry port,12,13 define flow dynamics in the true and false lumen, establish the mechanisms and severity of aortic regurgitation14 and assess ventricular function and the presence of tamponade (Figure 1).
Figure 1. Transesophageal echocardiography in aortic dissection. A. Intimal dissection in the ascending aorta (arrows). B. Large entry port (arrows) in the distal aortic arch. C. Prolapse of the intimal dissection seen through the aortic valve. D. Secondary communication port between the true and false lumen. AAo indicates ascending aorta; FL, false lumen; TL, true lumen.
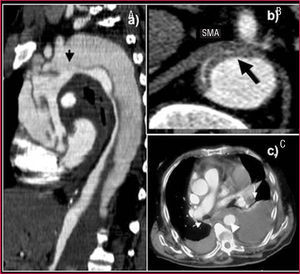
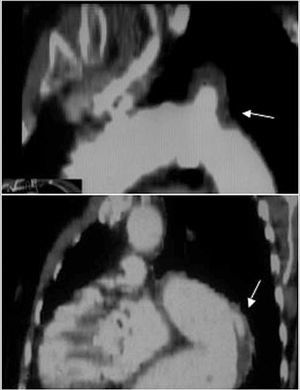
Nevertheless, it is inferior to CT in visualizing the entire aorta, diagnosing periaortic bleeding and artery branch disease (Figure 2). In 10% of aortic dissections the main tear is located in the abdominal aorta and are thus invisible to TEE. Contrast agents make it possible to demonstrate that flow is retrograde in the false lumen. Our group showed that false positive findings of ascending aortic dissection due to echoes in the aortic wall could be avoided by correctly analyzing the movement and location of the intima via M mode.15
Figure 2. Computerized tomography (CT) in aortic dissection. A. Spiral CT where a type A dissection is observed that starts in the aortic root and finishes in the abdominal aorta with total thrombosis of the false lumen. The arrow indicates the location of entry port. B. Severe compression of the true lumen hindering filling of the superior mesenteric artery. C. Pleural effusion and secondary hemomediastinum (arrows) as complications of an intramural hematoma (arrowhead). SMA indicates superior mesenteric artery.
Harmonic imaging has improved the sensitivity of TEE in visualizing intimal dissection, but does not make it possible to definitively rule out AAS. It is very useful as a complementary technique, since it diagnoses cardiac tamponade and quantifies aortic regurgitation. The best combination for correctly diagnosing acute aortic dissection and its complications is CT and transthoracic echocardiography16 (Table 1). Ascending aortic dissections should be examined via transesophageal echocardiography when serious diagnostic questions arise following CT or when hemodynamic instability makes it inadvisable to transfer the patient. In any case, the patient should be strongly sedated and hemodynamic monitoring constant during the procedure.
Visceral or peripheral malperfusion syndrome is a complication with high morbidity and mortality.17 Although echocardiography can be useful in diagnosing dissection of the supraaortic trunks, celiac trunk, and superior mesenteric artery, CT provides far more precise information. On the other hand, this technique is irreplaceable for diagnosing renal and iliac artery disease. There are two types of circulation disorders of the arterial branches: dissection or dynamic obstruction of the intimal dissection at the ostium of the arterial branches leaving the aorta18 (Figure 2B). Differentiating the two mechanisms has important therapeutic implications. Coronary arteries can be affected by aortic root dissection. Nevertheless, TEE is sufficient to diagnose this.13
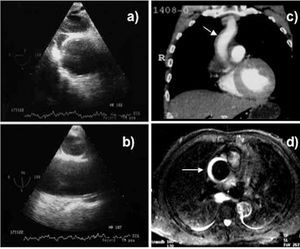
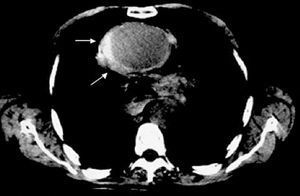
Although MRI is the most precise technique for diagnosing AAS,19 its use is open to question in the acute phase in patients with symptoms or hemodynamic instability where strict follow-up is required and who need immediate access to treatment. Its use would be justified in asymptomatic and stable patients where the diagnosis of AAS has not been established via other techniques as in intramural hematoma, for example. Unlike aortic dissection, bleeding of the vasa vasorum can be very progressive in intramural hematoma; in 15% of patients the former imaging technique does not reveal intramural bleeding, whereas the latter technique diagnoses intramural hematoma when done after 24-48 h7 (Figure 3). It should be recalled that diagnosing intramural hematoma via CT is done without contrast agent, since it is the low signal intensity from the wall that indicates the diagnosis11 (Figure 4). In the hyperacute phase, intramural bleeding is isodense on T1-weighted images and has high signal intensity on T2-weighted images. At 24-48 h, both T1 and T2 demonstrate high signal intensity.2
Figure 3. Progressive development of an intramural hematoma of the ascending aorta. Transesophageal echocardiography at 14 h from pain onset does not show alterations in the ascending aorta assessed in the transverse plane (A) and longitudinal plane (B). Spiral computerized tomography (C) at 2 days shows minimum dilatation in the ascending aorta (arrow) that was not considered significant. Magnetic resonance imaging at 5 days revealed a clear intramural hematoma due to the high-intensity signal in T2 (arrow).
Figure 4. Intramural hematoma in the ascending aorta (arrows) diagnosed via computerized tomography without contrast agent.
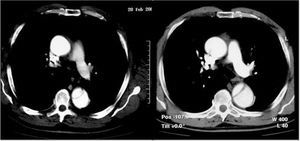
Less information is available on penetrating aortic ulcer as an AAS disease entity. Imaging techniques can diagnose various processes with different pathogeneses and prognoses. Contrast-enhanced imaging techniques, such as angiography and CT, have greater diagnostic sensitivity (Figure 5) when enabling the visualization of an ulcer protruding from the aortic lumen. Nevertheless, TEE is the technique of choice to classify the different types of ulcers regarding their pathogenesis and makes it possible to differentiate between ulcerated plaque found above the intima and atherosclerotic ulcers that penetrate the medial layer and frequently deform the adventitia. Finally, both CT and TEE are very useful for diagnosing ulcer-like images found in the evolution of aortic intramural hematoma as a consequence of a localized dissection.20
Figure 5. Aortic ulcers located at the beginning of the descending aorta diagnosed via computerized tomography. The upper image shows an atherosclerotic ulcer and the lower image an ulcer-like dissection in the subacute phase of an intramural hematoma.
In short, CT is the imaging technique with greater availability 24 h per day in most hospital centers and has excellent diagnostic accuracy (Table 2). If the diagnosis of type A dissection is confirmed, transthoracic echocardiography should be done to assess the presence and etiology of aortic regurgitation and rule out cardiac tamponade. A TEE should be done immediately before surgery, if possible with the patient already anesthetized, to confirm the previous findings and locate the dissection entry port. This technique is of great intraoperative help and, in any case, should always be done after the surgical intervention. Only in those cases where the information provided by CT is doubtful would TEE be indicated with diagnostic aims. If there is high clinical suspicion and predisposing cardiovascular factors and the first imaging technique was not diagnostic, an imaging technique should be repeated, preferably MRI when possible, at 48-72 h after symptom onset.
Biomarkers
Various biomarkers that can facilitate the diagnosis of AAS have been studied in recent years. The D-dimer test has demonstrated its usefulness in diagnosing AAS, especially after the first 6 h. One of its main drawbacks is that it does not enable the differential diagnosis of pulmonary thromboembolism.21 Myosin heavy chain markers have high sensitivity and specificity in the first 6 h, but their usefulness decreases at 12 h after symptom onset.22 Research into the behavior of these and other new biomarkers, such as homocysteine or metalloproteinases, may modify diagnostic strategies regarding AAS in the near future and be of great assistance in carrying out the differential diagnosis between the syndromes that are accompanied by chest pain, such as acute coronary syndrome and pulmonary thromboembolism.
EVOLUTION IN THE ACUTE PHASE. PROGNOSTIC VARIABLES
Type A Aortic Dissection
Various studies have confirmed greater early mortality in type A aortic dissection than in type B.4,5,8 Despite diagnostic and therapeutic progress, mortality during hospitalization for type A dissection continues to be high (>30%).4,23 The fact that two-thirds of the patients in this registry with type A dissection were sent from regional hospitals indicates that real mortality may be higher than 40%, since the most unstable patients who die in the regional centers or during transfer are not included. The presence of complications such as cardiac tamponade, a diseased coronary artery, compromised brain perfusion or heart failure due to severe aortic regurgitation implies very bad prognosis.8 Thus, in the IRAD registry, hypotension/shock/tamponade, kidney failure, sudden pain onset, pulse deficit, and age >70 years were independent predictors of mortality.23 The presence of periaortic hematoma is more frequent in type A dissections than in type B (26% vs 19%) and is an independent predictor of mortality (33% vs 20%; P<.001).24
Type A aortic dissection is a surgical emergency, although some authors25 consider that retrograde thrombosed type A dissections have better prognosis than anterograde, both in the acute phase (mortality 15% vs 38%) and at 5-year follow-up (mortality 15% vs 43%). They25 recommend treating retrograde thrombosed type A dissections medically and retrograde dissections with flow surgically. This strategy can be useful in patients older than 75 years or with comorbidity.
Type B Aortic Dissection
Patients with type B dissection without complications have 30-day mortality close to 10%.4 On the other hand, mortality exceeds 30% in patients with bad visceral or peripheral perfusion, kidney failure, or signs of contained rupture.17,18 The IRAD26 data indicate that 12% of patients with type B dissection present hypotension or shock, 16% have an aortic diameter greater than 60 mm, 19% have periaortic hematoma, and 22% present arterial vessel disease with bad perfusion, mainly in the ileac, mesenteric or renal arteries. Hospital mortality was 13%. A mortality risk model demonstrated the high-risk triad of hypotension/shock, absence of chest pain at clinical presentation and arterial branch disease. These variables indicated 3%, 36%, and 71% mortality,27 according to whether one, two or three risk factors were present. Clearly, unstable hemodynamics with severe hypotension or shock involved the highest risk. Other studies have shown that the variables age, compromised hemodynamics and peripheral ischemia indicate a worse prognosis in type B aortic dissection.28
Intramural Hematoma and Penetrating Atherosclerotic Ulcer
Intramural hematoma evolves very dynamically and can be reabsorbed, progress to a classic or localized dissection or present as contained rupture during the first days of evolution.29,30 Early mortality basically depends on the location and is higher when the ascending aorta is affected.31 However, there are serious differences in the published results, since mortality in medically treated type A hematoma was 36% in a metaanalysis of European and American publications,3 and less than 10% in most Asian groups.32,33 It is probable that in the Asian series, sensitivity in diagnosing intramural hematoma was much higher, given that intramural hematomas represented 20%-30% of AAS, whereas in IRAD this was only 5%.7 Including less important hematomas could account for a more benign profile carrying less risk of complications.
Although the therapeutic strategy for type A intramural hematoma should be the same as for aortic dissection,34 some studies have pointed out that certain morphological data imply a more benign profile than that of dissection. These morphological data are: maximum aortic diameter <50 mm, hematoma thickness <11 mm and absence of localized dissection.32-36 In stable patients with these morphological data, treatment could start with ß-blockers and non-emergency surgery performed given the best surgical conditions. In any case, age and the general state of the patient are the variables to consider since in the medium term it is probable that intramural bleeding will repeat and fail to evolve benignly.
Type B aortic intramural hematoma should be treated medically, except in patients with serious aortic dilatation (>60 mm), signs of imminent aortic rupture, or poor clinical and hemodynamic evolution. It is well-known that the incidence of periaortic hematoma and pleural effusion is greater in intramural hematoma than in dissecting hematoma.35 The diagnosis of these complications themselves is not an indication for surgical treatment providing there is no progression or they are not accompanied by poor prognosis factors.
Acute aortic symptomatic penetrating atherosclerotic ulcer has a risk of rupture equal to or higher than acute aortic dissection or intramural hematoma.37 Like intramural hematoma, it is much more frequently located in the descending aorta. In some cases, atherosclerotic ulcer evolves into saccular or fusiform aneurysm.38 In our experience, most penetrating atherosclerotic ulcers in the acute phase are diagnosed in the context of intramural hematoma. However, the diagnosis of this disease entity is not unusual in asymptomatic patients.
TREATMENT IN THE ACUTE PHASE
Medical Treatment
The patients with aortic dissection should receive immediate treatment to control the pain and reduce systolic blood pressure to <110 mm Hg. Morphine sulfate and intravenous ß-blockers (metoprolol, propanolol or labetalol) are usually used with this aim. If ß-blockers are contraindicated calcium antagonists can be used, such as verapamil or intravenous diltiazem. In patients with marked hemodynamic instability intubation, mechanical ventilation and TEE should be done. In cases where serious tamponade is diagnosed, a pericardiocentesis should be attempted with only partial evacuation of the liquid to avoid an increase in aortic bleeding, plus emergency surgery.
Type A Dissection
Emergency surgical treatment should be undertaken in type A aortic dissection, unless there are formal contraindications, especially if serious irreversible cerebral or visceral injuries have been established. The aim of surgery is to avoid aortic rupture or complications with life-threatening risk, such as tamponade or massive aortic regurgitation. When the dissection extends to the aortic arch it is difficult to achieve complete repair. Surgical mortality, even in centers of excellence, is high (15%-35%).4,23,24,39 In some series, surgical mortality is very low, but this is almost always due to patient selection or a more than 48-h delay between symptom onset and surgery. The main factor limiting emergency surgical treatment of type A dissection can be the surgeon's expertise. Obviously, complex surgery is involved requiring a high level of surgical expertise which the duty team cannot always offer.
Establishing cardiopulmonary bypass with anterograde arterial perfusion is recommended during surgical treatment, even using the right axillary route, confirming by TEE that the true lumen has been perfused. Retrograde femoral perfusion is not free from complications, involving embolization of atheromatous material, extension of the dissection or even bad organ perfusion. Surgery under total circulatory stoppage involves using scrupulous protection methods, with profound hypothermia and selective cerebral perfusion via the anterograde or retrograde route. Circulatory stoppages longer than 40 min without cerebral perfusion can lead to cerebral complications. Thus, currently, cerebral perfusion techniques with anterograde hypothermia are being increasingly used. In a recent study published by Ehrlich et al,39 31% of 167 patients who underwent surgery between 1987 and 2001 died or presented permanent neurological deficits, which was only related to hemodynamic instability (odds ratio [OR]=6.0). On the other hand, 16% had transitory neurological injuries related to being more than 60 years old and associated coronary disease.40
Surgical treatment basically consists in closing the dissection entry port, replacing the ascending aorta and aortic arch if required, having previously repaired and reconstructed the distal aorta. Aortic root repair and aortic valve preservation should always be attempted using the David resuspension technique. Nevertheless, if anatomical injuries to the root are very serious, it is better to completely replace it with a valved conduit with coronary artery reimplantation, to avoid reinterventions. Anastomosis of the most distal part of the prosthetic tube with the ascending aorta wall is appropriate whenever there is no entry port in the arch. The entry port is located in the arch in around 30% of patients and in such cases the arch should be replaced beyond the entry port. Replacing the arch can reduce the persistence of the distal patent false lumen from 80% to 40%. Whether there is an aneurysm in the proximal descending aorta, dissected or not, extending an elephant trunk from the arch is a good choice.41 A prosthetic tube placed in the descending aorta can be connected distally in a later procedure or, simultaneously, surgery of the ascending aorta undertaken combined with endovascular treatment in the descending aorta.42 The role of TEE is emphasized regarding optimizing the results of the various surgical strategies. This imaging technique provides precise information on the location of the entry port, pathogenesis of aortic regurgitation and disorders of the distal arch and descending thoracic aorta.
Type B Dissection
Complication-free type B dissection is treated via controlling blood pressure and cardiovascular risk factors. Surgical treatment is indicated when life-threatening complications appear: hemodynamic instability, intractable pain, rapid expansion of aortic diameter, mediastinal or periaortic hematoma with signs of imminent aortic rupture, or appearance of signs of bad perfusion of the most important arterial branches. Mortality in the acute phase in patients with renal ischemia is 50%-70%, and 80%-90% in those with mesenteric ischemia.43 Reviewing the scientific literature shows that surgical mortality in type B dissection is very high (28%-65%)42 and, in addition, there is a risk of paraplegia in around a third of the patients (30%-35%). In the IRAD registry,44 17% of type B dissections needed surgical treatment and hospital mortality was 29%.
In recent years, endovascular treatment has undergone strong development. In 1999, Dake et al45 and Nienaber et al46 simultaneously published their initial experiences of endovascular treatment in type B dissection. The aim of this procedure is to close the entry port, exclude flow from the false lumen and prevent evolution toward aneurysmatic dilatation and later aortic rupture. If the high surgical mortality in patients with bad perfusion of the arterial branches is considered, especially when early treatment is not undertaken, endovascular treatment is clearly indicated when there is static or dynamic obstruction of one of the arterial trunks.47,48 Static obstruction may be solved by placing a stent at the origin of the vessel, and dynamic obstruction by decompression of the false lumen by closing the entry port. In type A dissections, early restoration of reperfusion to vital organs makes it possible to stabilize the patient, and thus surgical repair can be done with greater chances of success.
Endovascular treatment can help to not only drastically reduce mortality in complicated type B dissection, but probably changes the natural history of this disease by promoting suitable remodeling of the aorta and reducing progressive dilatation of the false lumen. Mortality is <10% and risk of paraplegia <3%, thus systematic closure of the dissection entry port has been proposed in all the patients in the subacute phase of dissection. In our opinion, this option is not advisable in all cases, since nearly 50% of the patients present good long-term evolution.
Indications for percutaneous fenestration is not well-established and has decreased since endovascular treatment has become available. In patients with arterial ischemia due to the false lumen compressing the arterial ostium, it is vital to decompress the false lumen by fully closing the entry port via stent implantation or, if this is not possible, carry out fenestration of the infrarrenal aorta thus creating an exit port in the false lumen. Endovascular balloon fenestration is especially recommended in mesenteric ischemia situations47 where endovascular treatment is not possible. Endovascular ultrasonography can be very useful in obtaining optimal results with this technique.
Intramural Hematoma and Aortic Ulcer
The treatment of intramural hematoma is similar to that of aortic dissection. Hematoma of the ascending aorta requires surgical treatment, although if there are no complications, such as persistent pain, periaortic bleeding, or aortic ulcer, it can be operated on under the best surgical conditions in the first 24-72 h. In the patients older than 75 years or those with comorbidity, the next follow-up could be done with imaging techniques until it is demonstrated that the hematoma has been reabsorbed. The treatment of choice for hematoma of the descending aorta is medical. Evolution with localized dissection yielding ulcer-like images is not by itself an indication for surgical or endovascular treatment. On the other hand, the presence of serious rapid dilatation with signs of imminent rupture and periaortic bleeding in a patient with persistent pain indicates endovascular treatment. Such treatment can be undertaken with good results when the ends of the stent are implanted in the healthy aortic wall and not on the hematoma, since it could lead to intimal rupture on a weakened aortic wall.
Treatment of symptomatic penetrating atherosclerotic ulcer in the acute phase should be similar to that of other acute aortic syndromes, given the risk of aortic rupture.49 Outside the acute phase, treatment will depend on the evolution pattern, depending on the symptoms, progressive dilatation, or rebleeding of the aortic wall. When endovascular treatment was not available, surgery involved a certain risk in senile patients and those with atherosclerosis, and many aortic ulcers remained totally stable in the short-medium term. Nevertheless, we believe that endovascular treatment may be indicated in larger ulcers, especially if dilating or causing rebleeding on the aorta wall as diagnosed via magnetic resonance imaging.
EVOLUTION AND LONG-TERM TREATMENT
Medical treatment after the acute phase is fundamental. Correct control of blood pressure is associated with 96% survival free from aortic rupture at 5 years, compared to 61% in the poorly controlled group.42 Another important aspect is follow-up with imaging techniques to assess the appearance of a new dissection or aneurysm formation in other aortic segments. The incidence of such recurrences can be around 25%, and some evolve with complications, such as rupture and death by bleeding. Follow-up should include TEE or MRI before hospital discharge, an imaging procedure at 6 and 12 months, and annually thereafter, if the patient remains stable and asymptomatic. The TEE and MRI contribute functional information of great prognostic value and CT permits the correct follow-up of aortic diameters.
Type A Dissection
Even though surgery changes the natural history of type A aortic dissection, patients surviving surgical treatment can develop complications. These are basically associated with failure of surgical repair leading to progressive aortic root dilatation, with or without associated aortic regurgitation, or are secondary to persistent flow in the false lumen of the aorta distal to the prosthetic tube. The incidence of persistent false lumen flow distal to the prosthetic tube ranges between 79% and 90%50,51 and, although it cannot be considered a complication, long-term mortality and morbidity associated with this situation are high.51 Complication-free survival in surgically treated type A dissections is 84% at 5 years when the false lumen is totally thrombosed and 63% when flow becomes evident in the false lumen.51 Thus, eliminating the entry port, and not just repairing the ascending aorta, is of great importance in surgical intervention. In the IRAD registry, mortality at 3 years was 10% and the predictors of mortality were age, the presence of other atherosclerotic disease or heart surgery prior to the dissection.52
Type B Dissection
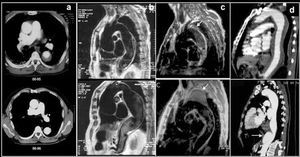
Long-term survival in type B dissection has been considered similar to that in surgically treated type A dissection ie, 75% at 5 years.5,8 Suitable medical treatment with ß-blockers is fundamental in preventing complications. ß-blockers delay aortic dilatation by reducing blood pressure and dP/dt. The dose should be progressively increased until blood pressure is <130/80 mm Hg. Some follow-up studies of type B dissection have reported an 18%-30% risk of aortic rupture at 3-5 years, and a 20%-30% need for surgical treatment.53,54 Factors predictive of such evolution are age, chronic obstructive pulmonary disease, hypertension, and the baseline maximum diameter of the aorta.55 In an interesting study, Kato et al56 showed that an aortic diameter >40 mm in the acute phase and the presence of a patent entry port in the thoracic aorta were markers of an aneurysm developing in the false lumen (>60 mm). In this group of patients, 30% had an aortic aneurysm at 3-year follow-up (Figure 6). Marui et al57 showed that there was serious dilatation or aortic rupture at 5-year follow-up in 67% of the patients with an aortic diameter >40 mm and patent false lumen, whereas this only occurred in 6% of the patients who did not present either of these variables in the acute phase. The high incidence of total thrombosis of the false lumen indicates that intramural hematomas, which have a different evolutionary pattern, were included in these studies.
Figure 6. A type B dissection followed up via computerized tomography. Study of acute aortic syndrome done at discharge (left image) and at 12 months (right image). Note the marked dilatation of the dissected aorta.
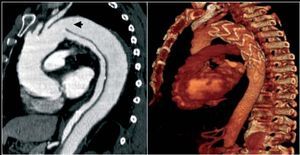
Although endovascular treatment is much more useful in the subacute phase of dissection (Figure 7), if a serious dilatation of the aorta >60 mm or growth >3 mm/year is found this should be implemented after the early phase. Recent studies demonstrate that endovascular treatment for dissection of the descending aorta is safe and offers better results than surgery.42,58 In the IRAD registry, mortality per year and per 3 years was 90% and 78%, respectively, with similar figures in the medical, surgical or endovascular treatment groups. Variables associated with mortality were the presence of other atherosclerotic disease, kidney failure, pleural effusion, or hypotension/shock in the acute phase.53
Figure 7. Left: computerized tomography during the acute phase shows a type B dissection with a large entry port (arrow) and moderate compression of the true lumen (TL). Since it presented signs of poor distal perfusion with persistent abdominal pain, endovascular treatment was implemented. Right: computerized tomography per year following the acute episode with complete remodelling of type B dissection.
Intramural Hematoma and Aortic Ulcer
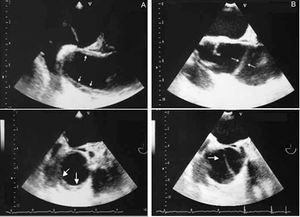
The long-term prognosis of type A hematoma is better than that of aortic dissection. In the series of Kaji et al,59 there was total resolution of the hematoma in 40% of 30 hematomas of the ascending aorta and there was no mortality at 4-year follow-up. In our experience,31 2 out of 8 patients treated medically evolved toward a complication-free dissection after 3-month follow-up (Figure 8). Thus, follow-up is required every 2 or 3 months with imaging techniques during the first year.
Figure 8. Transesophageal echocardiography visualizing the ascending aorta in the longitudinal (upper) and transverse (lower) planes. Intramural hematoma (A) in the ascending aorta (arrows) evolving into dissection at 3 months (B). The arrows indicate the intimal dissection.
In the Asian series, hematoma of the descending aorta also had a better prognosis than dissection, where 5-year survival was 97% in intramural hematoma versus 79% in dissection of the descending aorta.60 In our series, mortality in the patients with aortic hematoma was 19% at 3 months and 34% at 5 years, which is very similar to that in other non-Asiatic groups.31 Unlike aortic dissection, where most complications occur during the acute phase of the event, intramural hematoma can evolve in various ways, with possible complications during the subacute phase and in the first 6 months. Thus, the use of at least one or two imaging techniques is essential before hospital discharge and in the subacute phase. Few studies have assessed the morphological evolution of intramural hematoma in the mid- to long term. In our series38 of 50 intramural hematomas followed up with imaging techniques, evolution was very dynamic, mainly in the first 6 months: the intramural hematoma regressed without complications in 34% of cases, progressing to dissection in 36%, classical dissection in 12%, localized dissection in 24%, and evolving toward fusiform or saccular aneurysm in the remaining 30% (Figure 9). Aortic rupture is unusual if the maximum diameter is <60 mm and no new rebleeding of the aortic wall occurs. One of the most striking findings of the evolution of intramural hematoma is its evolution into localized dissection. Depending on its size, this lesion looks like an aortic ulcer or pseudoaneurysm. Many authors assess these images as being serious complications of intramural hematoma,36 but in fact some disappear without complications whereas others tend to dilate.38
Figure 9. Different types of evolution of a hematoma of the descending aorta. The acute episode (upper image) and at the end of follow-up (lower image). A. Total reabsorption without complications at 14 months after the acute episode. B. Fusiform dilatation of the entire descending aorta at 5-year follow-up. C. 70-mm saccular aneurysm (arrows) at 8-year follow-up after several episodes of asymptomatic bleeding visualized by magnetic resonance imaging. D. Localized dissections with formation of pseudoaneurysm 6 years after the acute episode.
Given the dynamic evolution of intramural hematoma and that 15%-20% of patients die from aortic rupture in the first 5 years, very close follow-up at 3, 6, and 12 months is recommended, and every year thereafter, until the intramural hematoma has been completely reabsorbed. If the evolution shows a progressive increase in aortic diameter or in the thickness of the hematoma with signs of aortic wall rebleeding, surgical or endovascular treatment is indicated.
CONCLUSIONS
Acute aortic syndrome continues to involve high mortality despite diagnostic and therapeutic progress during the last decade. Better understanding of the natural history and prognostic variables of this disease would be of great help regarding what is the most appropriate therapeutic strategy. However, early clinical suspicion and the expertise of the surgeon continue to be the basic factors associated with mortality in the acute phase. The introduction of endovascular treatment has opened up new perspectives on the treatment of acute aortic syndrome affecting the descending aorta; it is an excellent choice in the early treatment of complications and in most cases leads to aortic remodelling which can modify the natural history and improve the prognosis of this disease.
Section Sponsored by the Dr Esteve Laboratory
Correspondence: Dr. A. Evangelista Masip.
Servei de Cardiologia. Hospital Universitari Vall d'Hebron.
P.o Vall d'Hebron, 119-129. 08035 Barcelona. España.
E-mail: aevangel@vhebron.net