There is scarce real-world evidence on the management of perioperative antithrombotic treatment according to current recommendations. The aim of this study was to analyze the management of antithrombotic treatment in patients undergoing surgery or another invasive intervention and to assess the consequences of this management on the occurrence thrombotic or bleeding events.
MethodsThis prospective, observational, multicenter and multispecialty study analyzed patients receiving antithrombotic therapy who underwent surgery or another invasive intervention. The primary endpoint was defined as the incidence of adverse (thrombotic and/or hemorrhagic) events after 30 days of follow-up with respect to management of perioperative antithrombotic drugs.
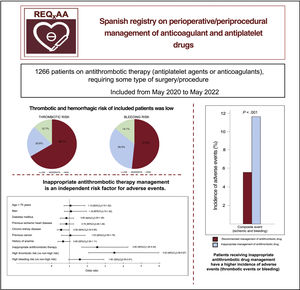
ResultsWe included 1266 patients (male: 63.5%; mean age 72.6 years). Nearly half of the patients (48.6%) were under chronic anticoagulation therapy (mainly for atrial fibrillation; CHA2DS2-VASC: 3.7), while 53.3% of the patients were under chronic antiplatelet therapy (mainly for coronary artery disease). Low ischemic and hemorrhagic risk was found in 66.7% and 51.9%, respectively. Antithrombotic therapy management was in line with current recommendations in only 57.3% of the patients. Inappropriate management of antithrombotic therapy was an independent risk factor for both thrombotic and hemorrhagic events.
ConclusionsThe implementation of recommendations on the perioperative/periprocedural management of antithrombotic therapy in real-world patients is poor. Inappropriate management of antithrombotic treatment is associated with an increase in both thrombotic and hemorrhagic events.
Keywords
The number of patients requiring surgery is increasing, and is now estimated at more than 300 million per year.1 Because of longer life expectancy, patients who undergo an invasive procedure are often under chronic antithrombotic therapy before the intervention.2 In Spain, around 1 million individuals take chronic anticoagulants, mainly for atrial fibrillation (AF), and the number is rising because of population aging and an increase in risk factors.3 In addition, there has been an upsurge in antiplatelet therapy for secondary prevention of atherothrombotic disease, in keeping with the increase in percutaneous coronary interventions (PCIs) and studies recommending prolonged use of these agents.4–7 To avoid periprocedural adverse events, it is important to determine whether and exactly when it is appropriate to interrupt antithrombotic therapy. The decision to withdraw or maintain these drugs is determined by the interaction between the patient's thrombotic risk and the bleeding risk associated with the intervention.8–10
In an attempt to simplify clinical decision-making, various guidelines and position papers have been published on perioperative management of antithrombotic drugs in several clinical scenarios.11–18 These include a consensus document on perioperative/periprocedural management of these drugs coordinated by the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology and certified by more than 20 Spanish scientific societies.19 Despite the existence of recommendations, little is known about their real-world impact on patients receiving anticoagulant or antiplatelet drugs and requiring an intervention. The aim of this study was to analyze antithrombotic therapy management in patients undergoing surgery or another invasive procedure, and evaluate the effect of interrupting or maintaining this therapy on the incidence of adverse cardiovascular and bleeding events.
METHODSThe REQXAA registry (Spanish registry on perioperative/periprocedural management of anticoagulant and antiplatelet drugs) is a prospective, observational, multicenter, multispecialty study with clinical follow-up of patients receiving antithrombotic therapy (antiplatelet agents or anticoagulants) and requiring surgery or another invasive procedure.
Study populationThe study enrolled patients aged ≥18 years on chronic treatment with at least 1 oral antithrombotic drug, including antiplatelet agents (eg, aspirin, clopidogrel, prasugrel, ticagrelor) or anticoagulants, either a vitamin K antagonist (VKA) (eg, acenocoumarol, warfarin) or a direct oral anticoagulant (DOAC) (eg, dabigatran, rivaroxaban, apixaban, edoxaban), and who underwent any type of intervention (surgery or another invasive procedure). Patients who did not give informed consent for the study and those who could not be followed up for 30 days after the procedure were excluded.
The demographic data, clinical history, laboratory values, and concomitant medication were obtained from the patients’ medical review and clinical records before the procedure. The patients’ thrombotic risk and the bleeding risk associated with the intervention were defined according to the recommendations of the above-mentioned Spanish consensus document.19 Briefly, thromboembolic risk was established based on the patient's clinical characteristics (CHA2DS2-VASC for AF, implanted prosthetic valve, and associated comorbidities), angiographic data (stent type), and time since the previous acute event (coronary syndrome, stroke, venous thromboembolic disease) that had indicated antithrombotic therapy. The procedure-associated bleeding risk was determined based on the potential consequences of any possible bleeding: high if it could be life-threatening or compromise surgical outcome, moderate if it could lead to an increased risk of transfusion or reoperation, and low if it would be potentially controllable (tables 1 and 2 of the supplementary data). The decision to continue or discontinue the antithrombotic drug was recorded, as was the time-point of discontinuation before the procedure, the use or nonuse of bridging therapy, and the time of resumption. Perioperative antithrombotic drug management was left to the discretion of the patient's attending physician. No interventions regarding the decision to maintain, interrupt, or resume this therapy were made by the study researchers. Clinical follow-up to record adverse events was carried out during the first 30 days after surgery. The study was initially designed to include all consecutive patients who met the inclusion/exclusion criteria during a 1 year period. However, due to the difficulties of patient enrollment and follow-up caused by the COVID-19 pandemic, the coordination committee decided to extend registration to 2 years.
The study was carried out in accordance with the Declaration of Helsinki, was approved by the Ethics Committee of Hospital Clínico San Carlos in Madrid, and was authorized by the other participating centers. All patients signed an informed consent for for study participation.
Primary endpointThe primary endpoint was the net incidence of a composite adverse event within 30 days after the intervention, consisting of all-cause mortality, nonfatal myocardial infarction, stent thrombosis, nonfatal ischemic stroke (focal neurological deficit caused by an ischemic event), peripheral embolism, venous thromboembolic disease, and/or grade> 2 bleeding complications according to the BARC20 classification. The secondary endpoint was the incidence of adverse events in relation to the suitability of the perioperative/periprocedural antithrombotic therapy, defined according to the recommendations of the Spanish consensus document.19 Briefly, the document proposes interrupting anticoagulant therapy for the shortest time possible based on the pharmacokinetics of the drug, limiting the use of bridging therapy to patients with high thromboembolic risk, maintaining periprocedural aspirin use in almost all interventions (with the main exception of neurosurgery) and assessing whether dual antiplatelet therapy should be continued in surgeries with low bleeding risk. Recommended/inappropriate management of antithrombotic therapy was determined by independent researchers using QXAApp, an online application developed to apply the recommendations of the Spanish consensus document.21
Statistical analysisThe Kolmogorov-Smirnov test was used to verify normal distribution of the variables analyzed. Categorical variables are expressed as the frequency and percentage. Quantitative variables are expressed as the mean±standard deviation if they met the normality condition or as the median [interquartile range] otherwise. Associations between categorical variables were verified using the chi-square or Fisher exact test when at least 25% of the values had an expected frequency <5. The Student t test was used to compare quantitative with dichotomous variables. Differences with a probability of error <5% (P <.05) were considered significant. A mixed-effects logistic regression model was used to identify all variables independently associated with occurrence of the composite adverse event (main outcome measure). The model included the research center as random effects, and the covariates consisted of statistically significant variables (P <0.20) and those considered clinically relevant; variables that could produce collinearity were avoided. The data are expressed as the odds ratio (OR) and 95% confidence interval. The statistical analysis was carried out using STATA, version 17.0 (Stata Corp, United States).
RESULTSBaseline characteristicsFrom May 2020 to May 2022, 1266 patients who had complete and valid perioperative/periprocedural data were included in the study (figure 1 of the supplementary data). Follow-up data for the first 30 days after the procedure were obtained in 1152 patients (91.0%). The baseline characteristics of the population are summarized in table 1. Most patients were men (63.5%), mean age was 72.6 years, and there was a considerable presence of comorbidities: hypertension (74.7%), diabetes mellitus (36.6%), and a history of ischemic heart disease (31.8%), AF (43.0%), heart failure (20.9%), and stroke (16.8%). Prior to the procedure, 615 patients (48.6%) were taking anticoagulants, mainly for AF (CHA2DS2-VASC, 3.7±1.6; HASBLED, 2.1±1.1), and 57.4% of these agents were DOACs. In addition, 676 patients (53.3%) were receiving antiplatelet agents, mainly for ischemic heart disease (597 were taking aspirin, at a dose <150mg in more than 95%). Finally, 103 patients (8.1%) were on dual antiplatelet therapy at the time of inclusion (mainly aspirin and clopidogrel) and 24 (1.9%) were taking concomitant antiplatelet and anticoagulant therapy.
Demographic analysis of the patients included (N=1266)
| Variable | |
| Age, y | 72.6±21.7 |
| Men | 804 (63.5) |
| Cardiovascular risk factors | |
| Active smoker | 245 (19.4) |
| Hypertension | 946 (74.7) |
| Dyslipidemia | 799 (63.1) |
| Diabetes mellitus | 463 (36.6) |
| Comorbidities | |
| Ischemic heart disease | 403 (31.8) |
| Stroke | 213 (16.8) |
| Peripheral vascular disease | 202 (16.0) |
| Heart failure | 265 (20.9) |
| Atrial fibrillation | 545 (43.0) |
| Mechanical valve | 61 (4.8) |
| Venous thromboembolic disease | 104 (8.2) |
| Chronic kidney disease | 213 (16.8) |
| COPD | 173 (13.7) |
| Cancer | 242 (19.1) |
| Liver disease | 57 (4.5) |
| Active alcoholism | 81 (6.4) |
| Anemia | 183 (14.5) |
| Previous bleeding | 87 (6.9) |
| Bleeding diathesis | 4 (0.3) |
| Antithrombotic therapy | |
| Aspirin | 597 (47.2) |
| Clopidogrel | 145 (11.5) |
| Prasugrel | 2 (0.2) |
| Ticagrelor | 25 (2.0) |
| Acenocumarol/warfarin | 262 (20.7) |
| Dabigatran | 49 (3.9) |
| Rivaroxaban | 71 (5.6) |
| Apixaban | 152 (12.0) |
| Edoxaban | 81 (6.4) |
| Laboratory data | |
| Hemoglobin, g/dL | 13.3±2.4 |
| Platelets, 1000/μL | 225.2±80.7 |
| CrCl, mL/min | 66.4±22.3 |
CrCl, creatinine clearance; COPD, chronic obstructive pulmonary disease.
Values are expressed as No. (%) or mean±standard deviation.
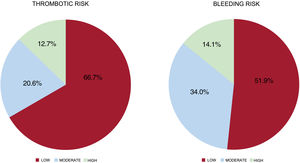
As to the type of intervention according to the medical specialty (table 3 of the supplementary data), 35.1% of the procedures were related to interventional cardiology or cardiovascular surgery, whereas the remaining procedures showed a more homogeneous distribution. Among the total, 66.7% of patients had a low thrombotic risk and 51.9% underwent procedures with a low associated bleeding risk (figure 1).
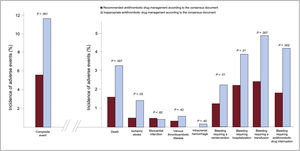
Perioperative/periprocedural antithrombotic therapyAntithrombotic therapy management was deemed appropriate according to the recommendations in only 57.3% of patients undergoing a procedure (62.1% of those receiving antiplatelet therapy and 49.1% of those receiving anticoagulants). Periprocedural bridging therapy was administered to 238 patients (18.8%), most of whom had been receiving anticoagulants: low molecular weight heparin in 231 (97.1%) patients, unfractionated heparin in 5 (2.1%), and an intravenous antiplatelet agent (tirofiban) in 2 patients (0.8%) who had been receiving dual antiplatelet therapy. Antithrombotic therapy management was inappropriate in 541 patients: the drug was interrupted earlier than recommended in 282 (52.1%) and later than recommended in 13 (2.4%), the drug was not withdrawn when recommended in 76 (14.0%), and bridging therapy was used inappropriately in 170 (31.4%). The group whose periprocedural antithrombotic therapy was considered inappropriate had a more frequent history of heart failure, AF, anemia, and bleeding. In addition, they were under chronic therapy with anticoagulant medication and received bridging therapy more often (table 4 of the supplementary data). The incidence of the composite adverse event was higher in patients with inappropriate management (11.7% vs 5.6%; P <0.001), as were the rates of thrombotic and bleeding events analyzed separately (figure 2).
Ischemic and bleeding events within the first 30 days following the procedure in relation to recommended or inappropriate antithrombotic drug management according to the Spanish consensus document.19
*Composite event: all-cause mortality, nonfatal myocardial infarction, stent thrombosis, nonfatal ischemic stroke, peripheral embolism, venous thromboembolic disease, and/or grade> 2 bleeding complications according to the BARC (Bleeding Academic Research Consortium)20 classification.
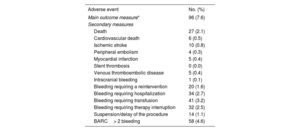
The incidence of the composite clinical event within the first 30 days after the intervention (main outcome measure) was 7.6% (96 patients) (table 2). Analysis of the epidemiologic and clinical characteristics of patients experiencing the composite event (table 3) showed a higher frequency of DM, ischemic heart disease, heart failure, chronic kidney disease, previous cancer, and anemia. In addition, they were receiving anticoagulants more often, had higher thrombotic and bleeding risks, and experienced greater use of bridging therapy and poorer use of periprocedural antithrombotic drugs.
Adverse events within the first 30 days after the intervention (N=1266)
| Adverse event | No. (%) |
|---|---|
| Main outcome measure* | 96 (7.6) |
| Secondary measures | |
| Death | 27 (2.1) |
| Cardiovascular death | 6 (0.5) |
| Ischemic stroke | 10 (0.8) |
| Peripheral embolism | 4 (0.3) |
| Myocardial infarction | 5 (0.4) |
| Stent thrombosis | 0 (0.0) |
| Venous thromboembolic disease | 5 (0.4) |
| Intracranial bleeding | 1 (0.1) |
| Bleeding requiring a reintervention | 20 (1.6) |
| Bleeding requiring hospitalization | 34 (2.7) |
| Bleeding requiring transfusion | 41 (3.2) |
| Bleeding requiring therapy interruption | 32 (2.5) |
| Suspension/delay of the procedure | 14 (1.1) |
| BARC> 2 bleeding | 58 (4.6) |
BARC, Bleeding Academic Research Consortium.
Main outcome measure defined as a composite of all-cause mortality, nonfatal myocardial infarction, stent thrombosis, nonfatal ischemic stroke, peripheral embolism, venous thromboembolic disease, and/or grade> 2 bleeding complications according to the BARC20 classification.
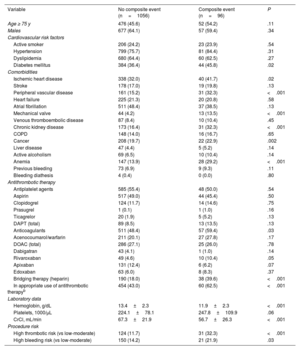
Analysis of the incidence of the composite event within 30 days following the procedure
| Variable | No composite event (n=1056) | Composite event (n=96) | P |
|---|---|---|---|
| Age ≥ 75 y | 476 (45.6) | 52 (54.2) | .11 |
| Males | 677 (64.1) | 57 (59.4) | .34 |
| Cardiovascular risk factors | |||
| Active smoker | 206 (24.2) | 23 (23.9) | .54 |
| Hypertension | 799 (75.7) | 81 (84.4) | .31 |
| Dyslipidemia | 680 (64.4) | 60 (62.5) | .27 |
| Diabetes mellitus | 384 (36.4) | 44 (45.8) | .02 |
| Comorbidities | |||
| Ischemic heart disease | 338 (32.0) | 40 (41.7) | .02 |
| Stroke | 178 (17.0) | 19 (19.8) | .13 |
| Peripheral vascular disease | 161 (15.2) | 31 (32.3) | <.001 |
| Heart failure | 225 (21.3) | 20 (20.8) | .58 |
| Atrial fibrillation | 511 (48.4) | 37 (38.5) | .13 |
| Mechanical valve | 44 (4.2) | 13 (13.5) | <.001 |
| Venous thromboembolic disease | 87 (8.4) | 10 (10.4) | .45 |
| Chronic kidney disease | 173 (16.4) | 31 (32.3) | <.001 |
| COPD | 148 (14.0) | 16 (16.7) | .65 |
| Cancer | 208 (19.7) | 22 (22.9) | .002 |
| Liver disease | 47 (4.4) | 5 (5.2) | .14 |
| Active alcoholism | 69 (6.5) | 10 (10.4) | .14 |
| Anemia | 147 (13.9) | 28 (29.2) | <.001 |
| Previous bleeding | 73 (6.9) | 9 (9.3) | .11 |
| Bleeding diathesis | 4 (0.4) | 0 (0.0) | .80 |
| Antithrombotic therapy | |||
| Antiplatelet agents | 585 (55.4) | 48 (50.0) | .54 |
| Aspirin | 517 (49.0) | 44 (45.4) | .50 |
| Clopidogrel | 124 (11.7) | 14 (14.6) | .75 |
| Prasugrel | 1 (0.1) | 1 (1.0) | .16 |
| Ticagrelor | 20 (1.9) | 5 (5.2) | .13 |
| DAPT (total) | 89 (8.5) | 13 (13.5) | .13 |
| Anticoagulants | 511 (48.4) | 57 (59.4) | .03 |
| Acenocoumarol/warfarin | 211 (20.1) | 27 (27.8) | .17 |
| DOAC (total) | 286 (27.1) | 25 (26.0) | .78 |
| Dabigatran | 43 (4.1) | 1 (1.0) | .14 |
| Rivaroxaban | 49 (4.6) | 10 (10.4) | .05 |
| Apixaban | 131 (12.4) | 6 (6.2) | .07 |
| Edoxaban | 63 (6.0) | 8 (8.3) | .37 |
| Bridging therapy (heparin) | 190 (18.0) | 38 (39.6) | <.001 |
| In appropriate use of antithrombotic therapyb | 454 (43.0) | 60 (62.5) | <.001 |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.4±2.3 | 11.9±2.3 | <.001 |
| Platelets, 1000/μL | 224.1±78.1 | 247.8±109.9 | .06 |
| CrCl, mL/min | 67.3±21.9 | 56.7±26.3 | <.001 |
| Procedure risk | |||
| High thrombotic risk (vs low-moderate) | 124 (11.7) | 31 (32.3) | <.001 |
| High bleeding risk (vs low-moderate) | 150 (14.2) | 21 (21.9) | .03 |
CrCl, creatinine clearance; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant.
aComposite event: all-cause mortality, nonfatal myocardial infarction, stent thrombosis, nonfatal ischemic stroke, peripheral embolism, venous thromboembolic disease, and/or grade> 2 bleeding complications according to the BARC (Bleeding Academic Research Consortium)20 classification. bAccording to the recommendations of the Spanish consensus document.19
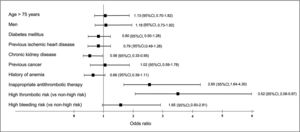
In relation to perioperative antithrombotic therapy, 60 of the 541 patients in whom this therapy was considered inappropriate experienced the composite event. In 31 (51.7%) bridging therapy was inappropriate, antithrombotic therapy had been withdrawn early/resumed late in 11 (18.3%), and the therapy was not interrupted in 11 (18.3%); there were no events in patients with late therapy interruption. A mixed-effects logistic regression model was used to assess the individual impact of these factors. The research center was included as random effects, whereas variables that were statistically significant in the univariate analysis and those considered clinically relevant were the covariates (figure 3). Thus, both high thrombotic risk (OR, 3.52; 95%CI, 2.08-5.97) and inappropriate periprocedural management of antithrombotic drugs (OR, 2.65; 95%CI, 1.64-4.30) were independent factors associated with the occurrence of the composite adverse event established as the main outcome measure.
DISCUSSIONThe results of the Spanish REQXAA registry, which provide an analysis of patients on chronic anticoagulant or antiplatelet therapy requiring surgery or another invasive procedure, indicate that real-world adherence to the current consensus recommendations for perioperative management of these drugs is low. Of particular note, patients whose periprocedural antithrombotic therapy management was deemed inappropriate had a more than 2-fold higher risk of experiencing an adverse ischemic or bleeding event after the intervention (figure 4).
The increase in patients on chronic therapy with anticoagulant and antiplatelet agents who may require an invasive procedure during their lifetime has led several scientific societies to publish consensus and position papers on the management of these drugs.11–18 However, many of the recommendations in these documents are limited by a lack of scientific evidence; hence, their application in clinical practice may be heterogeneous. It is important to determine not only the degree of adherence to these recommendation, but also whether proper adherence is associated with a lower incidence of adverse events following the procedure.
The literature contains very few registries analyzing local implementation of protocols for perioperative antithrombotic therapy. Rossini et al.13,22 evaluated the impact of an Italian consensus document for periprocedural management of antiplatelet therapy. Adherence to the Italian recommendations was as high as 85%, although the study was not designed to determine whether their application was associated with fewer cardiovascular events. This result contrasts with the findings of our registry, in which management was in accordance with the recommendations of the Spanish consensus document in only 57.3% of the patients. The percentage differed depending on the patients’ antithrombotic therapy: adherence was lower in patients receiving anticoagulants (49.1%) than in those using antiplatelet agents (62.1%). The finding that antithrombotic drug management was inappropriate according to the recommendations in almost half the patients is even more striking considering that the cohort's overall risk was low. Among the total, 12.7% of patients had a high thrombotic risk and 14.1% a high bleeding risk, and in general, the published recommendations for these patients are more controversial, as they require individualizing the antithrombotic therapy. There are several reasons for the differences observed. Dose adjustment is more difficult with anticoagulant drugs (eg, previous INR values with acenocoumarol or warfarin, renal function with DOACs) than with antiplatelet agents. In the latter case, especially in patients receiving aspirin monotherapy at a dose <150mg/d (most patients included in our registry) and with a previous PCI, most recommendations agree that this therapy should be maintained during the perioperative period.23,24
One of the main causes of inappropriate perioperative and periprocedural antithrombotic drug management is overuse of bridging therapy, which has been associated with a higher incidence of adverse events, in particular bleeding.25 Ferrandis et al.26 performed a study in patients receiving chronic DOAC therapy and requiring major surgery. Bridging therapy, administered to 35% of the patients, was associated with a higher incidence of bleeding events, with no difference in thrombotic events. These findings are supported by the results of a study by Douketis et al., 27 including more than 3000 DOAC-treated patients with nonvalvular AF who underwent surgery or another procedure. A strategy without bridging therapy used in that study yielded lower rates of thromboembolic and bleeding events. Evidence that bridging therapy may lack effectiveness has been reported in relation to some procedures, such as pulmonary vein ablation in AF patients and in certain situations outside the perioperative period (eg, following a stroke and before starting DOACs).28,29 These findings could add to the controversy around periprocedural antithrombotic management. In our registry, 18.8% of patients overall were administered perioperative bridging therapy, a value that rose to 35.3% in patients receiving anticoagulants.
In the present study, most patients had a low thrombotic and bleeding risk, but the 30-day incidence of the main outcome measure was 7.6%, which is not as low as might be expected. It is important to draw attention to this finding, in light of the large number of patients on antithrombotic therapy who require surgery at some point in their lives. In the statistical analysis, inappropriate perioperative antithrombotic management and high thrombotic risk were the 2 independent risk factors for some type of adverse event to occur within the first 30 days after the intervention. This finding underscores the importance of proper adherence to the recommendations, and not only in high-risk patients, to reduce the incidence of adverse events.
LimitationsThe main limitation of this study is that, like any observational analysis, it lacks an experimental design randomizing patients to a specific strategy for perioperative antithrombotic therapy management. Hence, the results on the incidence of adverse events and the implication of interrupting or maintaining this therapy should be interpreted with caution. In addition, as the study was conducted during the COVID-19 pandemic, there was some difficulty in obtaining all the patients’ data and study variables, including their 30-day follow-up. Despite these limitations, the study has the strengths of being a multicenter registry, in which professionals from several specialties contributed to patient recruitment, and the information was collected prospectively and systematically.
CONCLUSIONSThe results of the REQXAA registry have brought to light the limited real-world implementation of the consensus recommendations of various scientific societies for the management of perioperative antithrombotic therapy. Furthermore, inappropriate management in this context was found to be associated with an increased incidence of adverse ischemic and bleeding events. It is essential to insist on proper management of antiplatelet and anticoagulant therapy by adhering to local protocols to reduce perioperative complications.
FUNDINGNo funding received.
AUTHORS’ CONTRIBUTIONSD. Vivas and M. Anguita-Gámez contributed equally to the manuscript. All authors met the following requirements: 1) they contributed substantially to the conception and design of the study, and acquisition, analysis or interpretation of the data, 2) they participated in writing the article or critically reviewing its intellectual content, 3) they approved the final version to be published, and 4) they agreed to take responsibility for all aspects of the article and to investigate and resolve any issues related to the accuracy and veracity of the contents.
CONFLICTS OF INTERESTD. Vivas: presentation fees from Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Abbott, Boehringer Ingelheim, Bristol-Myers-Squibb and Ferrer. R. Ferrandis: presentation fees from LFB, CSL Behring and Octapharma. M. Anguita: presentation fees from Eli Lilly & Co, Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, Bristol-Myers-Squibb and Novartis; consultancy work for Eli Lilly & Co, Daiichi Sankyo, AstraZeneca, Bayer, Pfizer, Boehringer Ingelheim, Bristol-Myers-Squibb and Novartis. I. Egocheaga: presentation fees from Boehringer Ingelheim, AstraZeneca; consultancy work for AstraZeneca; support for conference attendance from Novartis. A. Abad-Motos: support for conference attendance from Vifor, Edwards. E. Figuero: presentation fees from Oral-B, Colgate, Johnson & Johnson, Spanish Society of Periodontology (SEPA), Irish Society of Periodontology, Ukrainian Association of Periodontology, French Dental Association of Periodontology; research grants from Dentaid, Lacer, Universidad de Bristol; support for attending conferences from SEPA, European Federation of Periodontology, Irish Society of Periodontology and French Dental Association of Periodontology. N. Bouzó-Molina: support for conference attendance from MSD. J. Torres: presentation fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly & Co, Novartis, Pfizer and Rovi. M.J. Descalzo: presentation fees from GlaxoSmithKline; support for conference attendance from Daiichi Sankyo. E. Martín-Rioboo: presentation fees from Servier, Ferrer, Boehringer Ingelheim. F. Marín: presentation fees from AstraZeneca and Boehringer Ingelheim; consultancy work for Boehringer Ingelheim; research grants from AstraZeneca, Ferrer and BMS; support for attending conferences from Esteve and Novo Nordisk; participation in advisory committees or monitoring of AFNET security data. All other authors declare they have no conflicts of interest.
AcknowledgementsThe Coordinating Committee of the REQXAA Registry would like to thank all the researchers (supplementary data) who participated in enrolling patients in the study.
- –
An increasing number of patients under chronic anticoagulant or antiplatelet therapy require an invasive procedure at some point in their lives.
- –
Numerous documents with recommendations on perioperative antithrombotic therapy management endorsed by scientific societies are available, but their real-world impact is unknown.
- –
Real-world implementation of recommendations on perioperative management of antithrombotic therapy is insufficient.
- –
Inappropriate perioperative/periprocedural management of antithrombotic therapy is associated with an increase in adverse thrombotic and bleeding events.
- –
It is essential to promote awareness and proper use of antithrombotic therapy during the perioperative/periprocedural period to reduce complications following interventions.
The authors guarantee that the following researchers are responsible for the data contained in this study:
Hospital Clínico San Carlos, Madrid. David Vivas (PI).
Hospital Universitari i Politècnic La Fe, Valencia. Raquel Ferrandis (PI).
Hospital Clínico Universitario Virgen de la Arrixaca, El Palmar, Murcia. Francisco Marín (PI).
Hospital Universitario Infanta Leonor, Madrid. Beatriz Nozal-Mateo (co-PI), Ane Abad-Motos (co-PI).
Facultad de Odontología, Universidad Complutense, Madrid. Elena Figuero (PI).
Facultad de Odontología, Universidad Rey Juan Carlos, Madrid. Rafael Gómez de Diego (PI).
Hospital Universitario Reina Sofía, Cordova. Manuel Anguita (PI).
Hospital Universitario 12 Octubre, Madrid. Nuria Bouzó-Molina (PI).
Hospital General Universitario Doctor Balmis, Alicante. Teresa Lozano (PI).
Hospital Universitario La Paz, Madrid. Carlos Álvarez (PI).
Hospital Universitario de Jaén. Javier Torres (PI).
Hospital Costa del Sol, Marbella, Málaga. María José Descalzo-Pulido (PI).
Hospital General Universitario, Valencia. Juan Carlos Catalá (co-PI), Francisco Ridocci (co-PI).
Unidad de Gestión Clínica de Atención Primaria Poniente, Cordova. Enrique Martín-Rioboo (PI).
Centro de Salud Isla de Oza, Madrid. Isabel Egocheaga (PI).
Centro de Salud Palma Norte, Madrid. Francisco Javier Torres-Martínez (PI).
Hospital Marina Baixa, Villajoyosa, Alicante. Alejandra Molines-Cantó (PI).
Hospital Universitario Torrecárdenas, Almería. Rocío Rodríguez-Contreras (PI).
Hospital Universitario La Zarzuela, Madrid. Juan José Sánchez-Palomo (PI).
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.recesp.2023.01.011