Keywords
INTRODUCTION
Pulmonary atresia with an intact septum (PAIS) is a serious cardiopathy that has an incidence of 0.7% to 3.1% of the congenital cardiopathies.1 It is characterized by great anatomical variability, where development of the right ventricle (RV) goes from a severely hypoplasic form and whose definitive treatment is a univentricular type correction, to the presence of a normal RV or with slight hypodevelopment with good possibility for a biventricular type correction. In addition to the degree of development of RV, tricuspid insufficiency (TI) is very variable with or without Ebsteins malformation and the coronary circulation can be totally or partially dependent on the RV.2-5 For these favorable cases with RV with acceptable development, and if the coronary distribution allows it, the treatment in the first days of life is valvular opening to favor the pulmonary flow and development of the right cavities. In 1991, Latson6 communicated the first case of valvular opening with the rigid tip of a guidewire, and in that same year, Qureshi et al7 and Parsons et al,8 by means of laser; in the following years, radio frequency with good results was also used.9-11 Our experience in valvular opening in PAIS began in 2001.12 Based on the mechanical techniques of Latson and with special guides for total chronic coronary obstruction on the other hand, we described a modified less aggressive form directed with a catheter loop in its anterograde form. since then we have carried out 11 consecutive cases of valvular opening in PAIS, with good initial results and with medium term follow-up, which is the subject of this study.
METHODS
Patients
Eleven newborn patient were seen (7 girls and 4 boys) with an average age of 9 (18) days (2-64 days), with weight varying from 2.5 to 3.4 kg and a body surface area (BSA) of 0.20 (0.02) m2.
All of them were diagnosed in our center and those with PAIS were referenced. They were admited to the pediatric intensive care unit and stabilized by means of the perfusion of prostaglandin E1 (PGE1); in spite of this, in 4 cases the procedure was performed as an emergency due to clinical instability. An echocardiographic study was carried out following the criteria of Kleinman13: evaluation of the pulmonary flow through a left pulmonary duct in all cases; presence or absence of ventriculocoronary sinus; size of RV and its formation in 1, 2, or 3 parts, and the competence of the pulmonary (PV) and tricuspid (TV) valves. The pulmonary ring and the pulmonary z, as well as the tricuspid ring in apical 4 chamber view and the z of the tricuspid were measured as an expression of the development of the RV,14,15 determining these values according to the normogramm proposed by Hanley et al3:
z value=measured diameter-average normal diameter/standard deviation of the normal average diameter
Valvuloplasty Technique
The procedure was previously performed according to the technique described6,12,16 by a femoral artery and vein puncture, with 4 and 5 Fr introducers. An aortography with the purpose of excluding a sinus dependent coronary circulation, as well as a right anteroposterior and lateral projections ventriculography, and positioning of the Goose-neck 5 mm loop (Microvena) through the duct was carried out on the PV. A coronary Judkins 3.5 catheter was employed in 7 cases and Goodale Lubin catheter in 4 cases, introduced straight to the RV and right under the PV "crowned" by the bow. The connection of the catheter was done with a coronary angioplasty "Y" key and, through this, we introduced special 0.014 inch guidewires for the total chronic coronary obstruction: using Crossit 200 and 300 (Guidant) in 7 cases; Choice P-T Graphix (Boston) in 3 cases and Asahi Confianza (Abbot) in 1 case. With the catheter supported on the PV, rotation maneuvers with a torque were made directing the guide to the loop and, once through it, it was extracted by the femoral artery, stablishing an arteriovenous circuit. With the stability that this circuit provides, we progressively achieved PV dilation with a low profile coronary monorail 2 and 4 mm and, once the valve was opened, we slid a Goodale Lubin catheter to the descending aorta, though which we advanced a 0.035 inches guide. With this guide we introduced 6 mm balloons, reaching a maximum balloon diameter between 8 and 12 mm. In 3 cases it was not possible to carry out this anterograde technique, leaving a sentry catheter in the infundibulum itself, retrieving the loop and its sheath, positioning it over the valve, perforating it in a retrograde form, capturing the guide with the loop in the vena cava and proceeding in similar form as in the previous technique, establishing the arteriovenous circuit. Finally, the angiographic control and the pressure reading are done in the usual way (Figures 1-5). After the procedure, the patients were again hospitalized in the neonatal intensive care unit, maintaining the PGE1 perfusion during 5 (4) days, with a progressive dose reduction during the following days. Follow-up was done through the pediatrics outpatient clinic of pediatric cardiology. Continuous variables were expressed as means (standard deviation) and the categorical ones as percentages. Student t test was applied for matched data and a P value less than .05 was considered significant. All calculations were performed using SPSS 11.01.
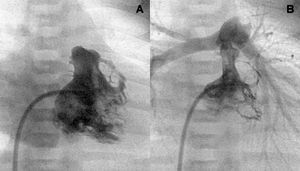
Figure 1. Right ventriculography. Tripartite ventricle with a good anterior and posterior valvular aperture development (A and B).
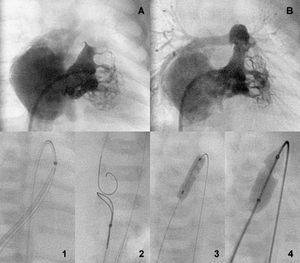
Figure 2. Bipartite ventricle before and after the aperture (A and B). Perforation a sequential anterograde valvuloplasty sequence (1-4).
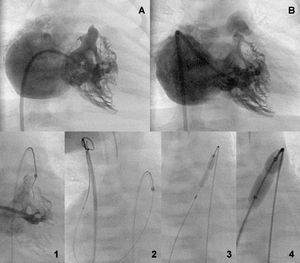
Figure 3. Bipartite ventricle before and after the valve aperture (A and B). Retrograde perforation sequence with guidewire capture by a loop in the inferior vena cava and sequential anterograde valvuloplasty (1-4).
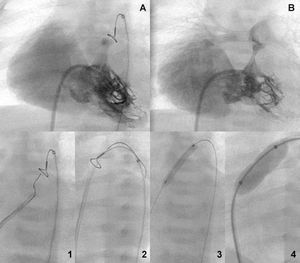
Figure 4. Tripartite ventricle with severe tricuspid insufficiency before and after the valvular aperture (A and B). Retrograde perforation sequence with capture of the guidewire through a loop in the superior vena cava and sequential anterograde valvuloplasty (1-4).
Figure 5. Tripartite ventricle and a Goose-Neck loop over the atresic valve (A). Anterograde perforation and progressive dilation (1-4). Valve aperture (B).
RESULTS
In the 11 cases, the presence of an infundibulum and ventriculopulmonary continuity data were confirmed through an echocardiogram. Eight had a tripartite RV and 3 a bipartite one due to obliteration of the trabecular segment; 2 had, additionally, a muscular subpulmonary obstruction and 1 a ventriculocoronary sinus, without anatomic obstructions and a preserved coronary flow on aortography.
With this technique, a maximum diameter of PV dilation with a balloon of 9.6 (1.2) mm (8-12) was achieved with a balloon/ring ratio of 143 (28)%, obtaining the following results:
1. The presence of a waist in the balloon catheters that progressively opened the PV.
2. Reduction in the RV pressure from 97 (17) to 48 (13) mm Hg (P<.001), with a final valvular gradient of 18 (16) mm Hg; in 2 cases a gradient greater than 40 mm Hg persisted and both presented a muscle-hypoplasia obstruction in the RV out flow tract.
3. Angiographic signs of PV aperture with a mobile valve and good contrast of the trunk and pulmonary branches.
4. Reduction in the degree of TI from 3.3 (0.9) to 2 (1.1) (P<.001) in the final ventriculography, measured in 4 degrees according to the density of the contrast of the right atrium.
5. Absence of immediate complications, tamponade, pericardial effusion or cardiac, or pulmonary artery perforation.
The anatomy of the RV and the hemodynamic data before and after the valvuloplasty are showed in the Table.
After the procedure, patients were taken to the neonatal intensive care unit and were reported stable. Nonetheless, one of them died after 24 h due to a pulmonary embolism, demonstrated through pulmonary scintigraphy and thrombosis in the lower right extremity as demonstrated through Doppler.
Over the course of the following days, the infusion of PGE1 was progressively reduced and in 6 cases (54%) no other procedure was done; in 4 cases (36%) additional pulmonary irrigation was needed in the first month: 2 central, a modified Blalock-Taussig and the implantation of a 4x18 coronary stent in the ductus was needed in another case. Patients were discharged after a hospital stay that lasted 30 (15) days.
During the 25 (21) months of follow-up (interval, 2-60 months), 2 patients with a previous fistula died: 1 of them at 2 months due to heart failure, undergoing a surgical closure of the fistula that evolved into an irreversible episode of shock; the other patient at 5 months, after being hospitalized for fever and pneumonia with a deteriorating evolution and, later, signs of low tissue perfusion; an urgent catheterisation was undertaken, showing a permeable fistula and the decision was taken to perform a Glenn intervention, but the patient presented a irreversible cardiac arrest during surgery.
In 2 symptomatic cases and in the presence of severe gradients of 50 and 60 mm Hg and a very hypoplasic bipartite RV at the age of 2 and a half years, a RV widening patch of the out flow tract surgery was and, in 1 of them, a bidirectional Glenn intervention (one and a half ventricle) was also done with a good outcome in both cases.
In this follow-up, the most relevant data was:
1. The survival of 72% of the children in very good functional conditions, achieving good development.
2. A reduction in RV pressure from 48 (13) mm Hg (hemodynamic) postvalvuloplasty to 33 (13) mm Hg (Doppler) (P<.05), including cases in which the obstruction was undone surgically.
3. An increase in the TV diameter from 10 (2) to 16.5 (2) mm (P<.01) and a reduction, though without significance, of the z score of the TV from 1.1 (1.3) to 1.3 (1) in spite of a good body development in the children.
4. Non-significant reduction of the TI degree from 2 (1) to 1.3 (0.7) (P=NS).
5. A growth in the PV ring from 6.7 (1.4) to 13 (1.8) mm (P<.001) and the pulmonary z from 2.2 (1.9) to 0.1 (1.7) though not significant (P=NS).
6. In all cases there was a residual pulmonary insufficiency observed that went from mild to moderate, quantified at 1.8 (0.5).
DISCUSSION
PAIS is a congenital cardiopathy with a great morphological diversity.1-5 This is demonstrated in the United Kingdom and Ireland collaboration study led by Daubeney et al.17 Of 183 cases, atresia was membranous in 74.7% and muscular in 25.3%; the RV was bipartite in 33.6% of cases and unipartite in 7%; coronary anomalies were identified in 45.8% of cases, with stenosis/interruption/ectasia in 7.6% and an Ebsteins malformation in 18 patients.
Anatomical and morphological characteristics of the RV are the main determinant in the therapeutic approach that is undertaken and the prognosis for the evolution of patients with this cardiopathy.17-19 Most of the authors consider that a biventricular repair cannot be viable when the z of the TV is less than 4. Wang et al20 estimates that treatment with a catheter can be definitive with a TV z of ≥0,1, pulmonary z ≥4.1, and a relation between ventricular areas of ≥0.65, and for Cheatham,14 when the TV ring is >11, the PV ≥7 mm and the RV volume is ≥30 mL/m2.
In an attempt to facilitate therapeutic approaches, Alwi21 recently published an algorithm on the therapeutic strategy in these patients: group A, patients with a tripartite RV, well developed and membranous atresia with a tricuspid z of >2.5 whose initial, and maybe definitive, treatment is valve opening through radiofrequency. Group C, patients with severe hypoplasia of a unipartite RV, with a tricuspid z of <5 where the initial treatment is an atrial septostomy, stent on ductus, or a modified Blalock-Taussig, and as something definitive, the bidirectional Glenn technique at 6-12 months, followed by a Fontan intervention. Group B or intermediate with a RV on the limit, a good infundibulum and a reduced trabecular segment with a tricuspid z of 2.5 to 4.5 whose initial treatment is valvulotomy, stent on ductus and a possible atrial septostomy; in this group it is also common to repair the out flow tract of the RV or the PV ring and the "one and a half ventricle" when the RV is not adequately developed.
In some studies, with the radiofrequency technique there is a greater effectiveness than with surgical valvulotomy and a demonstrated mortality,22 although in others this is not confirmed, but a smaller incidence of low output after this procedure19 has been demonstrated. After the first data on valvular opening in PAIS,6-10 the laser technique and, mainly, the radio frequency technique, experienced a great expansion, with several different series published.11,18,20,22-28 The results with this last technique in the revision of Benson et al29 show a success in 87% of the cases, with an incidence of complications of 15%, mortality of 8%, and the necessity of additional pulmonary flow in 33%. The main difficulties that have been described with the mechanical technique of Latson6 are the aggressiveness of the rigid tip of the guide, which can perforate the out flow tract and/or the pulmonary artery, as well as the unstable position of the subpulmonary catheter when the firm part of guide14 progresses. With this technique, the results have been inferior to those obtained with radiofrequency, with an initial success rate of 68%, mortality of 4%, and the necessity of additional pulmonary irrigation in 48%.14 In our experience with cases that in principle are favorable for valvular opening and that correspond to groups A and B of Alwi,21 we were able to open the valves in all of them and in a consecutive form during a period of 5 years. We used a modified Latson technique. We never used the rigid end of the guide, but special guides for total coronary chronic obstruction, always using the softer tip and directing to the loop in a supravalvular fashion. It allows for a greater stability of the catheter, that does not move from its position, and which is less aggressive, without any case of tamponade or pericardial effusions. The most effective guide in our experience has been the "Cross it" which, in addition to having a certain distal firmness, displays some sharpness in its tip from 0.014 to 0.010 inches, with capacity of lock and puncture when submitting to a rotational movement with the support of a catheter next to the membrane which is to be perforated, directing it to the loop, ductus, and the descending aorta. With respect to the catheter, we have observed that, for ample infundibula, the most stable is the right Judkins, and for narrow infundibula, the Goodale Lubin, with which one must be cautious when passing the guide through the distal orifice and avoid the 2 lateral ones. The exteriorized arteriovenous circuit that facilitates support, along with the low profile of the coronary monorail catheters, allows the initial valvular opening, proceeding in a progressive form, which has been mentioned above.12,16 When the anterograde technique was not possible, we would make it in an effective retrograde form, expanding progressively with balloon catheters in an anterograde manner. We considered that with the use of special guides for the total chronic coronary obstruction we have improved the results of the mechanical technique, being able to open the valves in 100% of the cases and with a procedural global success of 90%, a mortality of 9% and the need for additional irrigation in 36%. In the different medium and long term studies, there is a great variety of results that depend to a large extent on the anatomical variability and on the therapeutic approach that was followed.18,19,30 Mortality has been related to the aperture procedure, with the necessity for late or definitive surgery, and also inherent to the cardiopathy in itself, in its evolution. In the largest studies, survival is 60%-70% at 5 years19,30 and can be compared with ours of 72%, although with a shorter follow-up. Valvular opening allows a greater degree of flow by the TV, RV, and the PV, favoring the development of these structures; like in other studies, we observed that, although the PV and the TV increase significantly their diameter, this increase is not proportional to the body surface area and significative neither changes in the z score of the TV nor in the PV are appreciated; in some way, this indicates that the presence of a certain degree of hypodevelopment of the TV and the right cavities is not essential for maintaining a sufficient pulmonary flow.18 The limitations that this study may have are due to the small number of patients and the absence of a control group; nevertheless, its value when dealing with consecutive cases is reinforced. There might be some interobserver variability in the quantitative and semiquantitative measurements during the follow-up. In addition, it must considered that this hemodynamics study group makes pediatric and adult interventionism procedures simultaneously, with ample experience in coronary angioplasty, and their results should not be generalized.
CONCLUSIONS
1. Pulmonary valvuloplasty with a mechanical technique is still valid in PAIS.
2. The modification of the classic technique through the use of special guidewires for the total chronic coronary obstruction by its directed soft part is less aggressive and improves results.
3. In this series with favorable anatomy, the results are similar to those obtained through radiofrequency.
ADDENDUM
In the time this article was in print, 2 cases were treated successfully.
THANK YOU
To Drs Alberto Cabrera, José I, Arana, and Carlos Romero for their collaboration.
ABREVIATIONS
PAIS: pulmonary atresia with an intact septum
TI: tricuspid insufficiency
PGE1: prostaglandin E1
BSA: body surface area
LV: left ventricle
PV: pulmonary valve
TV: tricuspid valve
Correspondence: Dr. J. Alcíbar-Villa.
Sección de Hemodinámica. Hospital de Cruces.
Plaza Cruces, s/n. 48903 Baracaldo. Vizcaya. España.
E-mail: JUANCARLOS.ALCIBARVILLA@osakidetza.net
Received January 15, 2007.
Accepted for publication May 10, 2007.