Quantitative flow ratio (QFR) is a novel noninvasive method for evaluating coronary physiology. However, data on the QFR in patients with aortic stenosis (AS) and coronary artery disease are scarce. Thus, we compared the diagnostic performance of the QFR with that of the resting distal to aortic coronary pressure (Pd/Pa) ratio, fractional flow reserve (FFR), and instantaneous wave-free ratio (iFR), as well as angiographic indices.
MethodsA total of 221 AS patients with 416 vessels undergoing FFR/iFR measurements were enrolled in the study.
ResultsThe mean percent diameter stenosis (%DS) was 58.6%±13.4% and the mean Pd/Pa ratio, FFR, iFR, and QFR were 0.95±0.03, 0.85±0.07, 0.90±0.04, and 0.84±0.07, respectively. A FFR ≤ 0.80 was noted in 26.0% of interrogated vessels, as well as an iFR ≤ 0.89 in 33.2% and QFR ≤ 0.80 in 31.7%. The QFR had better agreement with FFR (intraclass correlation coefficient [ICC], 0.96; 95% confidence interval [95%CI], 0.95-0.96) than with the iFR (ICC, 0.79; 95%CI, 0.75-0.82) and Pd/Pa ratio (ICC, 0.52; 95%CI, 0.44-0.58). In addition, the QFR showed better diagnostic accuracy (98.6% vs 94.2%; P <.001) and discriminant function (area under the curve=0.996 vs 0.988; P <.001) when the iFR was used as the reference instead of FFR.
ConclusionsIn patients with AS, the QFR has good agreement with both FFR and iFR. However, the agreement appears to be even better when the iFR is used as the reference, presumably due to the complex nature of the coronary physiology in the assessment of coronary artery disease in patients with severe AS.
Keywords
Aortic valve stenosis (AVS) is accompanied by coronary artery disease (CAD) in up to 60% of patients undergoing surgical valve replacement or transcatheter aortic valve replacement (TAVR).1–3 Due to the multifactorial background of myocardial ischemia in AVS, assessment of the significance of intermediate coronary lesions in patients with AVS can be difficult. It should not only rely on visual angiographic assessment, but also involve more accurate evaluation of myocardial ischemia using invasive methods.4 Fractional flow reserve (FFR) and the instantaneous wave-free ratio (iFR) have been established as catheterization laboratory standards for that purpose, with proven impact on coronary revascularization and outcomes in patients without AVS.5–7 The resting distal coronary pressure to aortic pressure (Pd/Pa) ratio is a simpler index for the functional assessment of coronary stenosis that correlates well with FFR without the need for hyperemia induction.8 In addition, the quantitative flow ratio (QFR) is a novel tool based on computational fluid dynamics that is believed to virtually assess intermediate CAD.9,10 However, this new technique has not yet been validated in the setting of severe AVS. Thus, we compared the diagnostic performance of the QFR with that of the resting Pd/Pa ratio, FFR, and iFR, as well as angiographic indices, in patients with AVS and intermediate CAD.
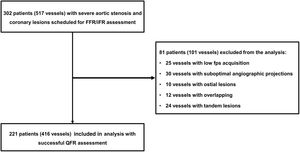
METHODSConsecutive patients with severe AVS undergoing routine coronary angiography with the presence of intermediate CAD (40%-90% diameter stenosis [%DS] by visual assessment) who were scheduled for FFR/iFR were prospectively enrolled between January 2018 and January 2020. Severe AVS was defined as a valve area <1.0 cm2 and mean aortic valve pressure gradient >40mmHg. The study was a prospective registry, oriented for QFR assessment. Study flow is presented in figure 1. Exclusion criteria were low fps acquisition, suboptimal angiographic projections, overlapping, ostial lesions not suitable for QFR assessment, and tandem lesions. Baseline clinical data were collected and assessed. All patients underwent wire-derived FFR/iFR assessment. The meticulous methodology of the FFR/iFR procedure was previously detailed.11–14 The iFR was assessed 3 times and the mean value was used in the current analysis. Adenosine was administered intravenously as a 140-μg/kg/min infusion for FFR measurement. The QFR was derived from 3D quantitative coronary angiography (Medis Suite 2.1.12.2, Medis Medical Imaging System, the Netherlands). QFR pullback with frame count analysis was performed separately on the 2 diagnostic angiographic projections without pharmacologically induced hyperemia. Independent core laboratory analyzers chose the QFR pullback with the best image quality (most well-defined contrast flow) in the frame count analysis. Angiographic, physiological, and QFR assessments were performed independently by 2 blinded core laboratory analyzers. Ethics approval was granted from the institutional ethics review process and all patients gave written informed consent.
Categorical variables are expressed as numbers of patients (percentages). Continuous variables are expressed as mean±standard deviation. Non-normally distributed data are reported as median (interquartile range [IQR]). The mean differences between the indices were calculated as absolute values. The agreement among the tested methods was assessed by the Bland-Altman plot method and intraclass correlation coefficient (ICC). The area under the curve (AUC) from receiver operating characteristic (ROC) analysis was used to assess the ability of the resting Pd/Pa ratio, QFR, and angiographic indices to predict a FFR ≤ 0.80 and iFR ≤ 0.89. Data are presented as the unadjusted AUC with 95% confidence interval (95%CI), and the DeLong method was used for comparisons. The diagnostic performances of the QFR are presented with sensitivity, specificity, and diagnostic accuracy and were compared using McNemar test or the weighted generalized score statistic. All tests were 2-tailed, and P <.05 was considered statistically significant. All statistical analyses were performed using STATISTICA 13.3 (TIBCO Software Inc, United States).
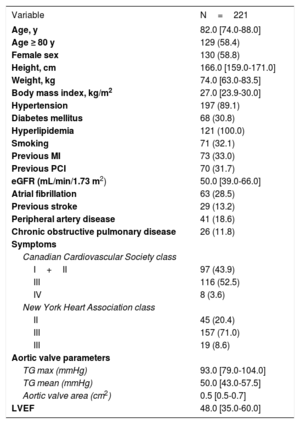
RESULTSWe assessed 416 borderline coronary artery stenoses in 221 patients with severe AVS (figure 1). Baseline patient characteristics are presented in table 1, whereas lesion characteristics are shown in table 2. The median age of enrolled patients was 82.0 years and 58.8% were women. The median aortic valve area was 0.5 cm2 and the aortic valve pressure gradient was 50 mmHg.
Baseline characteristics
| Variable | N=221 |
|---|---|
| Age, y | 82.0 [74.0-88.0] |
| Age ≥ 80 y | 129 (58.4) |
| Female sex | 130 (58.8) |
| Height, cm | 166.0 [159.0-171.0] |
| Weight, kg | 74.0 [63.0-83.5] |
| Body mass index, kg/m2 | 27.0 [23.9-30.0] |
| Hypertension | 197 (89.1) |
| Diabetes mellitus | 68 (30.8) |
| Hyperlipidemia | 121 (100.0) |
| Smoking | 71 (32.1) |
| Previous MI | 73 (33.0) |
| Previous PCI | 70 (31.7) |
| eGFR (mL/min/1.73 m2) | 50.0 [39.0-66.0] |
| Atrial fibrillation | 63 (28.5) |
| Previous stroke | 29 (13.2) |
| Peripheral artery disease | 41 (18.6) |
| Chronic obstructive pulmonary disease | 26 (11.8) |
| Symptoms | |
| Canadian Cardiovascular Society class | |
| I+II | 97 (43.9) |
| III | 116 (52.5) |
| IV | 8 (3.6) |
| New York Heart Association class | |
| II | 45 (20.4) |
| III | 157 (71.0) |
| III | 19 (8.6) |
| Aortic valve parameters | |
| TG max (mmHg) | 93.0 [79.0-104.0] |
| TG mean (mmHg) | 50.0 [43.0-57.5] |
| Aortic valve area (cm2) | 0.5 [0.5-0.7] |
| LVEF | 48.0 [35.0-60.0] |
eGFR, estimated glomerular filtration rate; LVEF, left ventricle ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; TG, transaortic gradient.
Data are expressed as no. (%) or median [interquartile range].
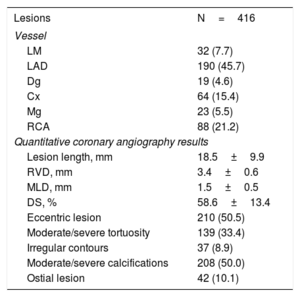
Lesion characteristics
| Lesions | N=416 |
|---|---|
| Vessel | |
| LM | 32 (7.7) |
| LAD | 190 (45.7) |
| Dg | 19 (4.6) |
| Cx | 64 (15.4) |
| Mg | 23 (5.5) |
| RCA | 88 (21.2) |
| Quantitative coronary angiography results | |
| Lesion length, mm | 18.5±9.9 |
| RVD, mm | 3.4±0.6 |
| MLD, mm | 1.5±0.5 |
| DS, % | 58.6±13.4 |
| Eccentric lesion | 210 (50.5) |
| Moderate/severe tortuosity | 139 (33.4) |
| Irregular contours | 37 (8.9) |
| Moderate/severe calcifications | 208 (50.0) |
| Ostial lesion | 42 (10.1) |
Cx, circumflex artery; Dg, diagonal artery; DS, diameter stenosis; LAD, left descending artery; LM, left main coronary artery; Mg, marginal branch; MLD, minimum lumen diameter; RCA, right coronary artery; RVD, reference vessel diameter.
Data are expressed as no. (%) or mean±standard deviation.
The study population comprised patients with coronary stenoses of intermediate angiographic severity (%DS 58.6%±13.4% by quantitative coronary angiography). The mean values of the Pd/Pa ratio, FFR, iFR, and QFR were 0.95±0.03, 0.85±0.07, 0.90±0.04, and 0.84±0.07, respectively. A FFR ≤ 0.80 was noted in 26.0% of interrogated vessels, as well as an iFR ≤ 0.89 in 33.2% and QFR ≤ 0.80 in 31.7%.
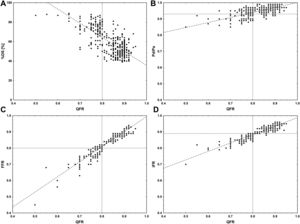
Limited intra- and interobserver variabilities in QFR measurement were confirmed, with ICCs of 0.991 (95%CI, 0.988-0.993) and 0.990 (95%CI, 0.987-0.992), respectively. The QFR had better agreement with FFR (ICC, 0.96; 95%CI, 0.95-0.96) than with the iFR (ICC, 0.79; 95%CI, 0.75-0.82) and Pd/Pa ratio (ICC, 0.52; 95%CI, 0.44-0.58). Scatter plots showing the relationship between the QFR and other angiographic and physiological indices are shown in figure 2.
Scatter plots showing the relationship between the quantitative flow ratio (QFR) and other angiographic and physiological indices. A, QFR and percent diameter stenosis (%DS); B, QFR and Pd/Pa ratio; C, QFR and fractional flow reserve (FFR); D, QFR and instantaneous wave-free ratio (iFR) Pd/Pa, resting distal to aortic coronary pressure.
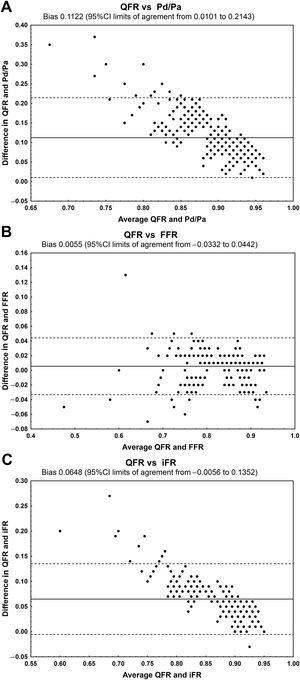
The QFR had very good agreement with FFR (mean difference, 0.0055; 95%CI,−0.0332 to 0.0442) and good agreement with the iFR (mean difference, 0.0648; 95%CI,−0.0056 to−0.1352) (figure 3). However, a clear bias was observed for the Pd/Pa ratio and iFR (not for FFR).
Bland-Altman analysis showing the mean absolute difference between the QFR and other physiological indices with 95% confidence limits. A, QFR and Pd/Pa; B, QFR and FFR; C, QFR and iFR. 95%CI, 95% confidence interval; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; Pd/Pa, resting distal to aortic coronary pressure; QFR, quantitative flow ratio.
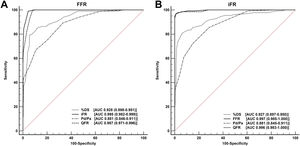
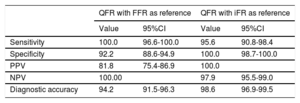
The QFR showed better diagnostic accuracy (98.6% vs 94.2%; P <.001) and discriminant function (AUC, 0.996 vs 0.987; P <.001) when the iFR was used as the reference rather than FFR. The discriminant functions of the iFR, QFR, resting Pd/Pa ratio, and %DS with a FFR ≤ 0.80 as reference standard were significant (P <.001 for all comparisons) and are presented in figure 4A. The discriminant functions of the FFR, QFR, resting Pd/P, and %DS with an iFR ≤ 0.89 as reference standard were significant (P <.001 for all comparisons), except for the QFR vs FFR (P=.38), and are presented in figure 4B. The diagnostic performances of the QFR are presented in table 3.
Overall diagnostic accuracy (area under the curve in receiver operating characteristic analysis) of quantitative flow ratio, Pd/Pa ratio, and percent diameter stenosis in detecting a fractional flow reserve ≤ 0.80 (A) and instantaneous wave-free ratio ≤ 0.89 (B). %DS, percent diameter stenosis; AUC, area under the curve; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; Pd/Pa, resting distal to aortic coronary pressure; QFR, quantitative flow ratio.
Comparison of the diagnostic performance and discriminant function of QFR with FFR and iFR used as reference standards
| QFR with FFR as reference | QFR with iFR as reference | |||
|---|---|---|---|---|
| Value | 95%CI | Value | 95%CI | |
| Sensitivity | 100.0 | 96.6-100.0 | 95.6 | 90.8-98.4 |
| Specificity | 92.2 | 88.6-94.9 | 100.0 | 98.7-100.0 |
| PPV | 81.8 | 75.4-86.9 | 100.0 | |
| NPV | 100.00 | 97.9 | 95.5-99.0 | |
| Diagnostic accuracy | 94.2 | 91.5-96.3 | 98.6 | 96.9-99.5 |
95%CI, 95% confidence interval; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; NPV, negative predictive value; PPV, positive predictive value; QFR, quantitative flow ratio.
Data are expressed as %.
The main findings of our study are: a) the QFR had better agreement with FFR compared with the iFR and Pd/Pa ratio; b) the QFR showed better diagnostic accuracy and discriminant function when the iFR was used as the reference; and c) the QFR may have the same limitations as other pressure-derived indices in the assessment of CAD in patients with severe AVS. Importantly, our study is one of the first to compare QFR performance with that of other hyperemic and nonhyperemic indices in CAD in the setting of severe AVS.
More than half of patients with severe AVS requiring surgical or percutaneous treatment have concomitant CAD.1–3 Severity assessment of CAD in this setting is difficult because invasive indices, both hyperemic and nonhyperemic, have not been fully validated in patients with severe AVS. Therefore, the evaluation of borderline CAD in patients with AVS remains an open question. Moreover, the avoidance of percutaneous coronary intervention in that subset of patients, often undergoing TAVR or even surgery, may improve clinical outcomes. The current guidelines on myocardial revascularization recommend revascularization in patients with AVS if the coronary stenosis involves proximal segments with more than 70% stenosis (by visual assessment) and in whom surgical valve replacement or TAVR is planned.15 The reason for this recommendation may be the complex coronary physiology in the setting of severe AVS. In this situation, intracoronary pressure gradients across lesions may be influenced by high left ventricular end-diastolic pressure resulting from a reduced valve orifice area or reduced coronary flow reserve due to microcirculation dysfunction in the presence or even absence of nonsignificant atherosclerotic plaques.16 Moreover, there is no evidence supporting physiology-guided coronary revascularization in patients with severe AVS.
Studies on coronary physiology assessment in the presence of severe AVS have yielded unexpected and contradictory results. Some studies found that the FFR values obtained are similar to those obtained after TAVR.17–19 On the other hand, some data suggested that FFR may underestimate intermediate coronary stenoses in the presence of severe AVS and that iFR values may be largely unchanged by AVS treatment, although there is little evidence on the use of iFR as reference.20–23 The wide adoption of FFR may remain problematic in this population because many operators may be cautious to use hyperemic drugs to avoid potential adverse hemodynamic effects in the presence of AVS. Additional costs, increased contrast load, and the prolongation of the diagnostic procedure may contribute to the low penetration of FFR/iFR assessment in elderly patients with AVS.
Thus, the QFR concept for assessing the severity of CAD may be highly attractive for overcoming these limitations and concerns. QFR, based on computational fluid dynamics, has been reported to be very accurate in the evaluation of intermediate CAD.10,24–27 However, data on QFR performance in the setting of severe AVS are scarce. In a study by Mejía-Rentería et al.,27 the QFR had good diagnostic performance in determining the FFR-based functional relevance of coronary stenoses in patients awaiting TAVR, with an AUC of 0.88 and classification agreement of 81%. In our study, interestingly, the QFR showed better diagnostic accuracy (98.6% vs 94.2%; P <.001) and discriminant function (AUC, 0.996 vs 0.987; P <.001) when the iFR was used as the reference (instead of FFR).
This finding may be due to various mechanisms, such as microvessel remodeling, left ventricle pressure overload, and hypertrophy leading to compression of small coronary vessels, particularly during the hyperemic state.16,28,29 In patients with severe AVS, despite higher ventricular pressures, the pressure in the proximal coronary artery in systole is lower than that arising from the distal artery due to the presence of a ventricular-aortic pressure gradient. Under these circumstances, the proximal coronary pressure is lower in diastole and the fall in pressure at the distal coronary artery is impaired due to different relaxation patterns in the hypertrophied left ventricle.16 Moreover, during stress, the ability of the AVS heart to increase coronary flow is limited because it is already working at near-maximal performance.16 Achievement of maximal hyperemia in the setting of AVS may be difficult in some patients and the accuracy of FFR may therefore be limited.30–32 The interaction among a severely stenosed aortic valve, elevated left ventricular end-diastolic pressure, left ventricular hypertrophy, and associated negative remodeling of the coronary microcirculation may theoretically blunt the response to adenosine, with a subsequent lack of maximal hyperemia.30–32 Moreover, the reliability of FFR in the assessment of myocardial ischemia assumes that pressure and flow are closely correlated with the microvascular resistances diminished during hyperemia.30–32 However, for these reasons, hyperemia may not be adequate in AVS patients.33
This is why we believe that nonhyperemic indices may be better for ischemia assessment in AVS patients and why the QFR (as a nonhyperemic index) showed better diagnostic concordance with the iFR. However, for the same reasons, the QFR may have the same limitations as invasive indices in patients with severe AVS.
In our study, we observed excellent concordance of the QFR with the FFR and iFR, even better than in the QASTA study by Mejía-Rentería et al.27 Their median reference vessel diameter was 2.8 mm (with 25% of vessels smaller than 2.5 mm), whereas it was 3.4 mm in our data, suggesting the presence of more lesions in more distal segments supplying less myocardium in the QASTA study vs more proximal locations in our study. Moreover, the mean %DS was 58.6% in our study but 48% in the QASTA study. Despite similarly mild physiological severity (FFR value, 0.84 vs 0.85), the percentages of lesions with a FFR <0.80 and QFR <0.80 were 26.0% and 31.7% in our study vs 40% and 46%, respectively, in the QASTA study. These aspects may be associated with the better QFR to FFR/iFR concordance in our results. In another study by Sejr-Hansen et al.,34 the diagnostic performance of the QFR before TAVR was tested and the acute post-TAVR FFR and iFR were used as references. The pre-TAVR QFR showed better diagnostic accuracy with the use of the post-TAVR FFR as a reference compared with the post-TAVR iFR (83% [95%CI, 68%-97%] and 52% [95%CI, 30%-74%], respectively; P=.008). In another study by Mejía-Rentería et al.35 analyzing 300 coronary arteries, the QFR exhibited decreased diagnostic performance compared with FFR in the presence of high microvascular resistance. On the other hand, no data are available on the assessment of CAD with a resting full-cycle ratio or Pd/Pa ratio or other nonhyperemic indices in the presence of AVS. Recently, Stähli et al.36 provided data on 516 vessels used to compare the diagnostic performance of the QFR and Pd/Pa ratio vs FFR. The QFR provided superior diagnostic accuracy compared with the resting Pd/Pa ratio and anatomical indices.
Moreover, another problem on the horizon concerns the treatment of patients with severe AVS. Revascularization of lesions with confirmed severity in these patients undergoing TAVR remains open to debate. The recently presented randomized ACTIVATION trial failed to support the use of revascularization in TAVR patients during work-up.37 Nonetheless, many questions remain to be answered to resolve the problem of lesion physiology assessment and the respective treatment in the setting of severe AVS.
Study limitationsOur study has several limitations. The coronary flow reserve was not assessed. Bland-Altman plots confirmed a clear bias for the comparison of the QFR vs the Pd/Pa ratio and iFR (not for FFR). The observed bias was probably related to the difference in the measurement technique. In general, the observed mean values were higher for the Pd/Pa ratio and iFR than for FFR, with less variability in Pd/Pa ratio and iFR values than in FFR values. Thus, the differences between these parameters might be much higher for significant lesions than for nonsignificant lesions. In addition, coronary physiology and QFR were not assessed after treatment of AVS.
CONCLUSIONSThe QFR has good agreement with both FFR and the iFR. Moreover, the diagnostic accuracy and discriminant function might be even better when the iFR is used as the reference, presumably due to the complex nature of coronary physiology in the assessment of CAD in patients with severe AVS. Moreover, the QFR may have the same limitations as invasive indices in patients with severe AVS.
- -
Several studies have attempted to resolve the unexpected and contradictory results of coronary physiology assessment in the presence of AVS.
- -
The QFR is a novel noninvasive method for evaluating coronary physiology.
- -
However, data on the QFR in patients with aortic stenosis and CAD are lacking.
- -
Only 2 similar studies have recently addressed QFR assessment in the setting of severe AVS.
- -
We compared the diagnostic performance of the QFR with that of the resting distal to aortic coronary pressure (Pd/Pa) ratio, fractional flow reserve (FFR), and instantaneous wave-free ratio (iFR), as well as angiographic indices.
- -
The QFR had better agreement with FFR than with the iFR and Pd/Pa ratio.
- -
In addition, the QFR showed better diagnostic accuracy and discriminant function when the iFR was used as the reference instead of FFR.
This study was supported by a grant from the National Science Center (application number: 2018/02/X/NZ5/02648).
AUTHORS’ CONTRIBUTIONSStudy design: P. Kleczynski. Data collection: P. Kleczynski, L. Rzeszutko, J. Legutko. Statistical analysis: A. Dziewierz. Data interpretation: P. Kleczynski, A. Dziewierz, J. Legutko. Manuscript preparation: P. Kleczynski, A. Dziewierz, L. Rzeszutko, D. Dudek, J. Legutko. Literature search: P. Kleczynski, J. Legutko. Funds collection: P. Kleczynski.
CONFLICTS OF INTERESTAll authors declare no conflicts of interest.