The Mitroflow aortic prosthesis is a bovine pericardial bioprosthesis specially designed to increase the valve area in relation to its size. There is controversy regarding the pattern of structural valve deterioration (SVD). Our aim was to determine the cumulative incidence of SVD, risk factors influencing its occurrence, and its impact on mortality.
MethodsA total of 1028 patients were clinically and echocardiographically followed up. Because the study population was elderly and had heart disease, we used a competing risk analysis.
ResultsThe percentage of patients with SVD at 5 years was 4.22% (95%CI, 2.96-5.81) and was 15.77% at 8 years (95%CI, 12.46-19.43). The incidence was higher for small valves (19mm and 21mm) reaching 6.43% at 5 years (95%CI, 4.48-8.84) and 20.06% at 8 years (95%CI, 15.53-25.01). Severe patient-prosthesis mismatch (PPM) influenced the incidence of SVD (sHR, 3.53; 95%CI, 2.20-5.66; P < .001) but moderate PPM had no impact. The most powerful predictor of mortality was the presence of SVD (HR, 4.59; 95%CI, 2.91-7.22; P < .001).
ConclusionsThis study used a definition based on the increase in the transprosthetic gradient and found a higher incidence of SVD of the Mitroflow prosthesis than that reported by other series, especially for sizes 19mm and 21mm and in patients with severe PPM. The incidence of SVD increased exponentially from the fifth year after implantation and its occurrence led to a 4.5-fold increase in the risk of death.
Keywords
Aortic valve stenosis is the most common condition affecting the cardiac valves and the third most frequent cardiovascular disease in the Western world, following hypertension and coronary artery disease. Severe aortic stenosis is seen in 2% of the population older than 65 years, and 4% of those older than 85 years.1 This disease is enormously relevant because of its high prevalence and ominous associated prognosis.
Currently, aortic valve replacement is the most frequently performed procedure in cardiac surgery departments in Spain, and an estimated 200 000 such procedures are performed worldwide per year.1,2
Biological prostheses account for more than 50% of all surgically implanted prosthetic valves in current use.1,2 Among these, the Mitroflow (Sorin Group Inc, Mitroflow Division; Vancouver, Canada) bovine pericardial prosthesis, available since 1982, is specifically designed to achieve an optimal hemodynamic profile.3,4 The results with the first model (11A) have been clouded by the development of tears in the leaflets resulting from contact with the valve skeleton.5 This shortcoming was corrected in the following 2 models (12A and LX), and the new design achieves a hemodynamic profile superior to that of other biological prostheses.3,4 In Mitroflow, the pericardial leaflets are externally mounted around a small stent, a configuration that maximizes the valve area relative to the size of the device.6 These characteristics, together with its adaptability and ease of supra-annular implantation, make the Mitroflow biological aortic valve an ideal prosthesis for use in patients with a small, calcified aortic annulus.4 This prosthetic valve is commonly used in Spain and has been implanted in more than 100 000 patients throughout the world.5
The main drawback of biological prostheses is structural valve degeneration (SVD). Although patient-related factors (eg, age, renal function, parathyroid metabolism) have a proven impact on the incidence of SVD, factors related to the prosthesis (eg, design and manufacturing process) are also associated with this late complication.7
Some authors have reported satisfactory mid- and long-term outcomes with Mitroflow.4,8–11 However, in other studies, a worrisome percentage of SVD has been observed,5,12 and some authors have predicted a global epidemic of patients with degenerated Mitroflow prostheses.5 The lack of anticalcification treatment in the 12A and LX models has emerged as the main hypothesis to explain the excessively early occurrence of SVD.5,12
Based on the assumption that all patients will develop the event of interest if the follow-up time is sufficiently prolonged, all the related studies have used Kaplan-Meier (KM) estimates to calculate SVD incidence.4,5,8–12 In this type of analysis, patients who do not survive are considered to be “censored”, falsely assuming that their prostheses could degenerate in the future.13 In fact, numerous competing events (CEs) could occur that would impede the development of the event under study. These conventional statistical methods4,5,8–12 assume that CEs do not exist, which invariably leads to overestimation of the true cumulative incidence of the event of interest.14
The primary aim of this study was to determine the cumulative incidence of SVD associated with Mitroflow 12A and LX aortic valve prostheses and the risk factors predicting its occurrence. The secondary aim was to determine the impact of SVD on survival.
METHODSSampleThe study included all patients who underwent conventional aortic valve replacement with a Mitroflow prosthesis (12A or LX) in a tertiary referral center in Spain between January 2006 and December 2014. The patients’ baseline characteristics, and the preoperative and postoperative data were prospectively recorded. The type of prosthesis used, the surgical approach, and the use of cardioplegia were at the discretion of the treating surgeon. Patients underwent a complete median sternotomy with supra-annular prosthesis implantation.
Definition of Structural Valve Degeneration and Other VariablesIn the absence of a standard criterion to define SVD, we used the following echocardiographic critera5: progressive transaortic pressure gradient ≥ 30mmHg associated with aortic valve area ≤ 1cm2 or intraprosthetic regurgitation > 2/4, provided it was not present within 30 days following the procedure and was not a consequence of endocarditis. All cases of SVD and all inconclusive cases were evaluated by 2 experienced echocardiographers, independent from the study.
The definitions related to morbidity and mortality during follow-up were set in accordance with the recommendations of the American Association for Thoracic Surgery and the European Association for Cardio-Thoracic Surgery ().15
The prosthesis-patient mismatch (PPM) cases diagnosed were classified according to the effective orifice area index value as moderate (≤ 0.85cm2/m2) or severe (≤ 0.65cm2/m2). The effective orifice area index was calculated by dividing the prosthetic valve area, obtained in the echocardiographic study prior to discharge, by the patient's body surface area, calculated using the Dubois formula.16 For patients who died during the postoperative period, we used the in vivo effective orifice area index values published in the literature.17
Follow-upAll patients surviving the postoperative period underwent echocardiography before hospital discharge. Long-term follow-up information was ensured by compiling all the clinical and echocardiographic data generated in our hospital and in the patients’ other referral centers (hospitals, primary care centers) for the study. Patients with inconclusive echocardiography findings or data raising the suspicion of SVD were referred for a new echocardiography and reevaluation at our center. Patients whose last echocardiogram had been performed more than 1 year previously and those showing elevated gradients or some degree of aortic regurgitation were also referred for a new echocardiographic examination. In patients without SVD, the last echocardiogram performed was used in the analysis. In those with SVD, we used the mean interval between the first echocardiogram revealing SVD and the last one in which it was not present (midpoint imputation). The day of aortic valve replacement was considered day 0.
Statistical Analysis. Competing EventsQuantitative variables are expressed as the mean ± standard deviation, and the median [interquartile range] is included in some cases to provide additional information. Categorical variables are expressed as no. (%).
Given that the study population comprised of elderly patients with heart disease, expected to have an elevated percentage of deaths during follow-up, we decided to carry out a competing risk analysis. There is unanimous agreement that this method, reported by Fine and Gray in 1999,18 correctly estimates the probability of an event of interest (SVD) occurring in the presence of other events (death) that would impede the development of the event under study.13,14 When the KM survival curve or the cumulative incidence curve based on KM analysis (1-KM) is used, CEs are “censored” and the estimated probability of the event occurring is invariably overestimated. In addition, the effect of covariates on the event of interest cannot be interpreted. When the analysis is performed taking CEs into account, the probability is correctly estimated and the model has a simple and practical clinical interpretation.14
Competing events were defined as all those impeding the development of the event of interest (in this case SVD), as follows: cardiac death (including patients who died during the postoperative period and excluding those who had SVD), noncardiac death, and valve replacement for reasons other than SVD. Patients who were alive at completion of follow-up with the same implanted valve and no evidence of SVD were the only ones considered “censored”.
Only 19 patients died without undergoing echocardiography during the year before their death. As it was impossible to know whether these patients had SVD at the time of death, they were considered lost to follow-up and were excluded. Five patients did not sign informed consent agreeing to a new echocardiographic examination.
Using CE analysis,18 we calculated the cumulative incidence of SVD and 95% confidence intervals (95%CI) at 3, 5, and 8 years. Furthermore, a subdistribution proportional hazards model (ie, regression analysis of the cumulative incidence function) was computed to investigate the impact of specific covariates on the SVD incidence.14 Because of the common use of KM analysis in the literature and to enable comparison with the results of other studies, the cumulative incidence curves based on the 1-KM estimator and the specific-cause incidence model were also analyzed. The latter was computed in the same manner as the Cox proportional hazards model, treating CEs as “censored”.14
The following covariates were entered in both regression models as possible predictors of SVD: age, creatinine clearance, dyslipidemia, diabetes, sex, and PPM.
To determine the impact of SVD on mortality, we carried out a Cox regression analysis using stepwise exclusion (inclusion, P < .05; exclusion, P ≥ .10) with the following covariates: age, sex, diabetes mellitus, dyslipidemia, hypertension, peripheral vascular disease, creatinine clearance, reduced mobility, previous cardiac surgery, previous chronic pulmonary disease, ventricular dysfunction (left ventricular ejection fraction ≤ 50%), severe pulmonary hypertension (pulmonary artery systolic pressure > 55mmHg), coronary disease, aortic surgery, mitral surgery, and SVD. This last factor was entered in the model as a time-dependent variable and its influence on mortality was calculated from the time of its development. The proportional hazards assumption was assessed by visual inspection of ln-minus-ln survival plots and by Schoenfeld residuals analysis.
Statistical analyses were carried out using STATA v.14.1 (STATA Corp; Texas, United States).
The study was approved by the Research Ethics Committee of the Principality of Asturias, under reference number 2916.
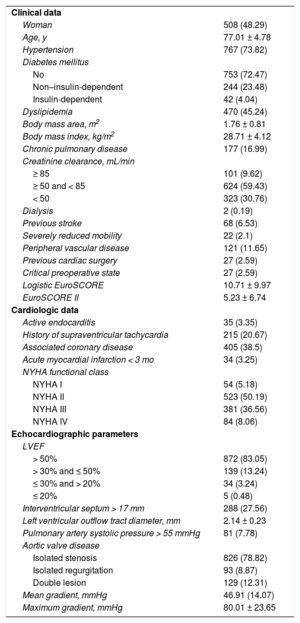
RESULTSBaseline Characteristics, and Intraoperative and Postoperative DataDuring the enrollment period, 1052 patients received a Mitroflow 12A or LX prosthesis. Mean age was 77.01 ± 4.78 years, and median age was 77.33 years (74.11-80.48): 28% were older than 80 years and only 6% were younger than 70 years. The age of the youngest patient was 34 years, and the oldest, 91 years (). The mean logistic EuroSCORE was 10.71 ± 9.97 and the EuroSCORE II was 5.23 ± 6.74. Only 54 patients (5.18%) were asymptomatic, and 826 (78.82%) were treated for aortic stenosis. Baseline characteristics are shown in Table 1.
Baseline Characteristics and Preoperative Data
| Clinical data | |
| Woman | 508 (48.29) |
| Age, y | 77.01 ± 4.78 |
| Hypertension | 767 (73.82) |
| Diabetes mellitus | |
| No | 753 (72.47) |
| Non–insulin-dependent | 244 (23.48) |
| Insulin-dependent | 42 (4.04) |
| Dyslipidemia | 470 (45.24) |
| Body mass area, m2 | 1.76 ± 0.81 |
| Body mass index, kg/m2 | 28.71 ± 4.12 |
| Chronic pulmonary disease | 177 (16.99) |
| Creatinine clearance, mL/min | |
| ≥ 85 | 101 (9.62) |
| ≥ 50 and < 85 | 624 (59.43) |
| < 50 | 323 (30.76) |
| Dialysis | 2 (0.19) |
| Previous stroke | 68 (6.53) |
| Severely reduced mobility | 22 (2.1) |
| Peripheral vascular disease | 121 (11.65) |
| Previous cardiac surgery | 27 (2.59) |
| Critical preoperative state | 27 (2.59) |
| Logistic EuroSCORE | 10.71 ± 9.97 |
| EuroSCORE II | 5.23 ± 6.74 |
| Cardiologic data | |
| Active endocarditis | 35 (3.35) |
| History of supraventricular tachycardia | 215 (20.67) |
| Associated coronary disease | 405 (38.5) |
| Acute myocardial infarction < 3 mo | 34 (3.25) |
| NYHA functional class | |
| NYHA I | 54 (5.18) |
| NYHA II | 523 (50.19) |
| NYHA III | 381 (36.56) |
| NYHA IV | 84 (8.06) |
| Echocardiographic parameters | |
| LVEF | |
| > 50% | 872 (83.05) |
| > 30% and ≤ 50% | 139 (13.24) |
| ≤ 30% and > 20% | 34 (3.24) |
| ≤ 20% | 5 (0.48) |
| Interventricular septum > 17 mm | 288 (27.56) |
| Left ventricular outflow tract diameter, mm | 2.14 ± 0.23 |
| Pulmonary artery systolic pressure > 55 mmHg | 81 (7.78) |
| Aortic valve disease | |
| Isolated stenosis | 826 (78.82) |
| Isolated regurgitation | 93 (8.87) |
| Double lesion | 129 (12.31) |
| Mean gradient, mmHg | 46.91 (14.07) |
| Maximum gradient, mmHg | 80.01 ± 23.65 |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association
The data are expressed as no. (%) or mean ± standard deviation.
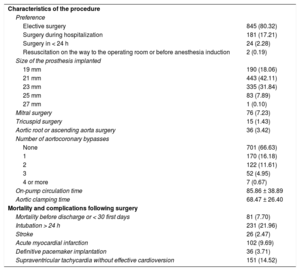
Nonelective surgery was carried out in 207 patients (19.68%), and 351 patients (33.36%) underwent associated myocardial revascularization surgery. Eighty-one patients (7.70%) died during hospitalization or within the first 30 postoperative days. The main cause of death was cardiogenic shock, occurring in 29 (35.80%) patients. The procedure characteristics and postoperative complications are shown in Table 2.
Intraoperative and Postoperative Results
| Characteristics of the procedure | |
| Preference | |
| Elective surgery | 845 (80.32) |
| Surgery during hospitalization | 181 (17.21) |
| Surgery in < 24 h | 24 (2.28) |
| Resuscitation on the way to the operating room or before anesthesia induction | 2 (0.19) |
| Size of the prosthesis implanted | |
| 19 mm | 190 (18.06) |
| 21 mm | 443 (42.11) |
| 23 mm | 335 (31.84) |
| 25 mm | 83 (7.89) |
| 27 mm | 1 (0.10) |
| Mitral surgery | 76 (7.23) |
| Tricuspid surgery | 15 (1.43) |
| Aortic root or ascending aorta surgery | 36 (3.42) |
| Number of aortocoronary bypasses | |
| None | 701 (66.63) |
| 1 | 170 (16.18) |
| 2 | 122 (11.61) |
| 3 | 52 (4.95) |
| 4 or more | 7 (0.67) |
| On-pump circulation time | 85.86 ± 38.89 |
| Aortic clamping time | 68.47 ± 26.40 |
| Mortality and complications following surgery | |
| Mortality before discharge or < 30 first days | 81 (7.70) |
| Intubation > 24 h | 231 (21.96) |
| Stroke | 26 (2.47) |
| Acute myocardial infarction | 102 (9.69) |
| Definitive pacemaker implantation | 36 (3.71) |
| Supraventricular tachycardia without effective cardioversion | 151 (14.52) |
The data are expressed as no. (%) or mean ± standard deviation.
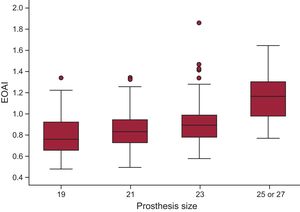
Three patients (0.31%) had intraprosthetic aortic regurgitation ≥ 2/4, and 3 others (0.31%) had periprosthetic regurgitation ≥ 2/4 immediately after the procedure. Moderate PPM was found in 374 patients (35.55%) and severe PPM in 104 (9.89%). The distribution of the effective orifice area index values for each prosthesis size is shown in Figure 1.
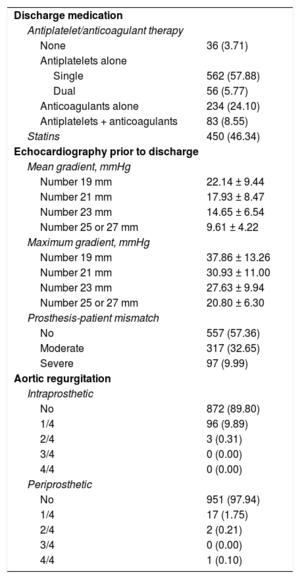
Echocardiography findings and treatment at hospital discharge are shown in Table 3.
Echocardiography and Treatment at Discharge in Patients Surviving the Postoperative Period
| Discharge medication | |
| Antiplatelet/anticoagulant therapy | |
| None | 36 (3.71) |
| Antiplatelets alone | |
| Single | 562 (57.88) |
| Dual | 56 (5.77) |
| Anticoagulants alone | 234 (24.10) |
| Antiplatelets + anticoagulants | 83 (8.55) |
| Statins | 450 (46.34) |
| Echocardiography prior to discharge | |
| Mean gradient, mmHg | |
| Number 19 mm | 22.14 ± 9.44 |
| Number 21 mm | 17.93 ± 8.47 |
| Number 23 mm | 14.65 ± 6.54 |
| Number 25 or 27 mm | 9.61 ± 4.22 |
| Maximum gradient, mmHg | |
| Number 19 mm | 37.86 ± 13.26 |
| Number 21 mm | 30.93 ± 11.00 |
| Number 23 mm | 27.63 ± 9.94 |
| Number 25 or 27 mm | 20.80 ± 6.30 |
| Prosthesis-patient mismatch | |
| No | 557 (57.36) |
| Moderate | 317 (32.65) |
| Severe | 97 (9.99) |
| Aortic regurgitation | |
| Intraprosthetic | |
| No | 872 (89.80) |
| 1/4 | 96 (9.89) |
| 2/4 | 3 (0.31) |
| 3/4 | 0 (0.00) |
| 4/4 | 0 (0.00) |
| Periprosthetic | |
| No | 951 (97.94) |
| 1/4 | 17 (1.75) |
| 2/4 | 2 (0.21) |
| 3/4 | 0 (0.00) |
| 4/4 | 1 (0.10) |
The data are expressed as no. (%) or mean ± standard deviation.
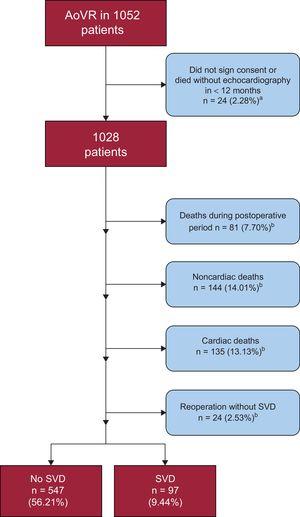
Among a total of 1052 participants, 24 patients were excluded from the analysis because they did not have a valid echocardiogram or they failed to sign the consent form. In the remaining 1028 patients, mean follow-up was 49.16 ± 30.31 months and median follow-up was 49 (25-70) months.
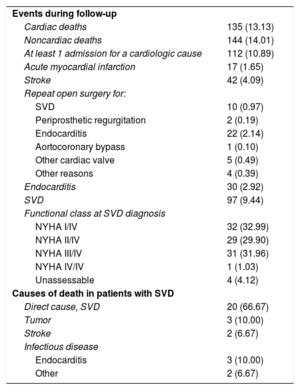
During the follow-up period, there were 135 (13.13%) cardiac-related deaths with no evidence of SVD, 144 (14.01%) noncardiac deaths, and 24 (2.33%) prosthesis replacements for reasons other than SVD (Figure 2); 22 (91.67%) of these were for endocarditis and 2 for severe periprosthetic regurgitation.
We performed 511 new echocardiography examinations exclusively for the purposes of the study, and analyzed 5693 echocardiograms. On average, 4.39 ± 3.31 echocardiograms were analyzed per patient following hospital discharge. Ninety-seven patients (9.44%) had SVD, and their mean age at diagnosis was 81.03 ± 5.43 years. The mean pressure gradient on echocardiography was 52.87 ± 17.67mmHg (with a variation of 19 to 81mmHg) and the maximum gradient was 88.42 ± 21.06mmHg (with a variation of 25 to 151mmHg). Most patients (n = 48; 49.48%) showed both progressive gradient increases and intraprosthetic regurgitation. In contrast, 9 patients (9.28%) had isolated aortic regurgitation. Among these 97 patients, 32 (32.99%) were completely asymptomatic at the time of the diagnosis (Table 4). Only 29 patients (29.89%) underwent a second intervention: 19 valve-in-valve procedures and 10 surgical procedures.
Events During Follow-up and Causes of Death in Patients With Structural Valve Degeneration
| Events during follow-up | |
| Cardiac deaths | 135 (13.13) |
| Noncardiac deaths | 144 (14.01) |
| At least 1 admission for a cardiologic cause | 112 (10.89) |
| Acute myocardial infarction | 17 (1.65) |
| Stroke | 42 (4.09) |
| Repeat open surgery for: | |
| SVD | 10 (0.97) |
| Periprosthetic regurgitation | 2 (0.19) |
| Endocarditis | 22 (2.14) |
| Aortocoronary bypass | 1 (0.10) |
| Other cardiac valve | 5 (0.49) |
| Other reasons | 4 (0.39) |
| Endocarditis | 30 (2.92) |
| SVD | 97 (9.44) |
| Functional class at SVD diagnosis | |
| NYHA I/IV | 32 (32.99) |
| NYHA II/IV | 29 (29.90) |
| NYHA III/IV | 31 (31.96) |
| NYHA IV/IV | 1 (1.03) |
| Unassessable | 4 (4.12) |
| Causes of death in patients with SVD | |
| Direct cause, SVD | 20 (66.67) |
| Tumor | 3 (10.00) |
| Stroke | 2 (6.67) |
| Infectious disease | |
| Endocarditis | 3 (10.00) |
| Other | 2 (6.67) |
NYHA, New York Heart Association; SVD, structural valve degeneration
Data are expressed as no. (%).
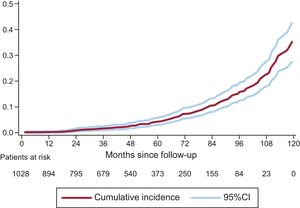
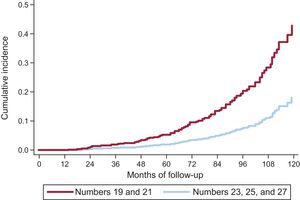
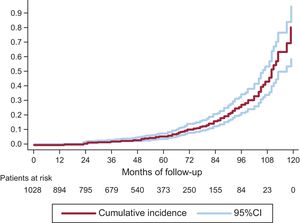
On competing risk analysis, the cumulative incidence of SVD was 1.59% (95%CI, 0.93-2.56) at 3 years, 4.22% (95%CI, 2.96-5.81) at 5 years, and 15.77% (95%CI, 12.46-19.43) at 8 years (Figure 3). The incidence was higher for smaller-sized valves (19 and 21mm): 2.63% (95%CI, 1.53-4.20) at 3 years, 6.43% (95%CI, 4.48-8.84) at 5 years, and 20.06% (95%CI, 15.53-25.01) at 8 years. A comparison of SVD incidence between prostheses 19 or 21mm in size and the remaining ones is shown in Figure 4. In 25% of patients at the younger end of the age range (age ≤ 74.11 years), the incidence of SVD at 3, 5, and 8 years was 2.14% (95%CI, 0.81-4.64), 5.75% (95%CI, −3.08 to −9.62), and 22.33% (95%CI, 15.59-29.83).
On KM analysis (Figure 5), the cumulative incidence of SVD was 1.77% (95%CI, 1.05-2.98) at 3 years, 5.19% (95%CI, 3.66-7.33) at 5 years, and 27.36% (95%CI, 21.58-34.31) at 8 years. The results of the 2 analyses showed considerable differences ().
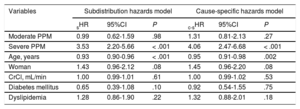
In the subdistribution hazards model, severe PPM (subhazard ratio [sHR], 3.53, 95%CI, 2.20-5.66; P < .001) and age (sHR, 0.93, 95%CI, 0.90-0.96; P < .001) were found to have an influence on the development of SVD. Similarly, in the cause-specific hazards model, severe PPM (cause-specific hazard ratio [c-sHR], 4.06, 95%CI, 2.47-6.68; P < .001) and age (c-sHR, 0.95, 95%CI, 0.91-0.98; P = .002) proved to be risk factors for SVD (Table 5).
Results of the Subdistribution Hazards Model and Cause-specific Hazards Model. Risk Factors for Developing Structural Valve Degeneration
| Variables | Subdistribution hazards model | Cause-specific hazards model | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | c-sHR | 95%CI | P | |
| Moderate PPM | 0.99 | 0.62-1.59 | .98 | 1.31 | 0.81-2.13 | .27 |
| Severe PPM | 3.53 | 2.20-5.66 | < .001 | 4.06 | 2.47-6.68 | < .001 |
| Age, years | 0.93 | 0.90-0.96 | < .001 | 0.95 | 0.91-0.98 | .002 |
| Woman | 1.43 | 0.96-2.12 | .08 | 1.45 | 0.96-2.20 | .08 |
| CrCl, mL/min | 1.00 | 0.99-1.01 | .61 | 1.00 | 0.99-1.02 | .53 |
| Diabetes mellitus | 0.65 | 0.39-1.08 | .10 | 0.92 | 0.54-1.55 | .75 |
| Dyslipidemia | 1.28 | 0.86-1.90 | .22 | 1.32 | 0.88-2.01 | .18 |
95%CI, 95% confidence interval; CrCl, creatinine clearance; c-sHR, cause-specific hazard ratio; PPM, prosthesis-patient mismatch; sHR, subhazard ratio.
During follow-up, 112 patients (10.89%) were readmitted due to cardiologic causes; 42 (4.09%) had stroke and 17 (1.65%) myocardial infarction. Sixty-three patients (6.13%) underwent a new cardiac surgery. The main reason was endocarditis (n = 22, 2.14%), followed by SVD (n = 10, 0.97%) (Table 4).
Of the 97 patients with a diagnosis of SVD, 30 (30.92%) died: 10 (33.33%) due to causes unrelated to SVD and 20 (66.67%) in direct relation to the presence of SVD. In these latter patients, 5 deaths occurred following surgical or percutaneous aortic valve implantation, and 15 deaths were due to heart failure refractory to treatment or sudden death ().
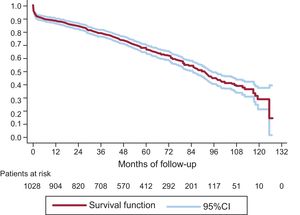
On KM analysis 3-, 5-, and 8-year survival rates were 79.31% (95%CI, 76.65-81.71), 67.69% (95%CI, 64.46-70.69), and 45.16% (95%CI, 40.62-49.59), respectively (Figure 6).
The Cox model showed that age (HR, 1.03; 95%CI 1.01-1.06; P = .009), diabetes mellitus (HR, 1.39; 95%CI, 1.12-1.74; P = .003), chronic pulmonary disease (HR, 1.67; 95%CI, 1.31-2.13; P < .001), reduced mobility (HR, 2.14; 95%CI, 1.09-4.18; P = .027), creatinine clearance (HR, 0.99; 95%CI, 0.98-0.99; P = .001), peripheral vascular disease (HR, 1.50; 95%CI, 1.13-1.98; P = .005), ventricular dysfunction (HR, 1.53; 95%CI, 1.18-1.97; P = .001), and SVD (HR, 4.59; 95%CI, 2.91-7.22; P < .001) were risk factors for death during follow-up.
DISCUSSIONStructural degeneration is the most important drawback associated with biological valve prostheses. The main finding of this study is that 4.2% of the Mitroflow prostheses analyzed showed SVD at 5 years and 15.8% at 8 years. In smaller prostheses (19 and 21mm), the percentages were even higher: 6.4% and 20.1% at 5 and 8 years, respectively.
Incidence of Structural Valve Degeneration and Risk FactorsAn exponential acceleration in the development of SVD was observed starting from the fifth year after implantation (Figure 3), a finding that should lead to particular vigilance at completion of this period. This acceleration was seen in relation to both large and small prostheses, but was particularly marked in the smaller ones (19 and 21mm).
There is no standard definition of SVD. Several studies analyzing SVD-free survival have defined this event based on a repeat procedure for this cause.19 In the present study, only 30% of patients with SVD underwent a new procedure, and other authors have reported an even lower percentage.5 The use of this definition leads to a clear underestimation of the true incidence of this event. Some authors10 have defined SVD in the same way as severe native valve aortic stenosis, while overlooking that severe PPM would perfectly fit this definition.
Sénage et al.5 used a new, more reasonable definition for SVD in a study including 617 patients with a Mitroflow prosthesis over a mean follow-up period of 3.8 years. Their main finding was an SVD incidence of 0.8% at 3 years and 8.4% at 5 years. In our study with 1028 patients analyzed over a mean follow-up of 4.1 years and using the same definition, we found an SVD incidence of 1.6%, 4.2%, and 15.8% at 3, 5, and 8 years, respectively. Thus, there was a lower incidence of SVD in our patients than the values reported by these authors,5 but the incidence was higher than would be expected according to the findings for other bioprostheses. In this line, the SVD-free survival rate at 10 years was reported to be 86.8% by Burguignon et al.20 98.5% by Anselmi et al.21 and 100% by Celiento et al.19 Nonetheless, these authors used different definitions,19–21 which makes it impossible to determine whether the Mitroflow valve undergoes degeneration sooner than other prostheses.
Several authors have indicated that PPM is one of the factors with the greatest impact on the development of SVD.5,22 The present study supports this notion but shows that moderate PPM has no impact; the effect is exclusively restricted to severe PPM. Paradoxically, the Mitroflow prosthetic valve was specifically designed to reduce the percentage of patients with PPM.3,4 Hence, there must be other causes to explain the high percentage of SVD. In their study, Sénage et al.5 speculated on these reasons, but the authors acknowledge that their relatively short follow-up prevented them from drawing conclusions. One hypothesis would be the absence of anticalcification treatment (in which case the incidence of SVD would increase exponentially after the fifth year) and another would be the presence of a sporadic structural defect during the first years of follow-up (in which case the incidence would increase linearly). The cumulative incidence curve we obtained clearly demonstrates an exponential acceleration in the risk of SVD starting from the fifth year following aortic valve replacement, which points to the first hypothesis as the main cause. Nonetheless, we believe that the combined effect of various factors cannot be excluded. First, the architecture of the prosthesis, with the pericardial leaflets externally mounted on the stent, would favorably increase the valve area, but could lead to pressure being mainly absorbed by the leaflets rather than the skeleton of the device. Second, the turbulent flow inherent to PPM could lead to fibrosis and calcification in a valve that has not been properly treated with anticalcification agents.
Impact of Structural Valve Degeneration on MortalityThis study shows that once SVD has developed, the patient's risk of death increases by 4.5-fold, and at this time, SVD is the main factor affecting prognosis. In response to this situation, it seems mandatory to intensify monitoring and convene a multidisciplinary team to decide on the most suitable medical-surgical strategy to apply. European guidelines23 recommend treating all symptomatic patients and all low-risk asymptomatic patients with SVD. In the present series, only 30% of patients were treated. A more assertive approach or earlier treatment might have reduced the impact of SVD on mortality. However, demonstrating this possibility is beyond the scope of the study and should be investigated in the future.
Competing EventsNone of the published reports4,5,8–12 on SVD associated with the Mitroflow prosthesis have used an analysis that adequately addresses CEs. Koller et al.24 have demonstrated the presence of these events in most studies with long-term follow-up published in scientific journals with the highest impact factors. Nonetheless, CEs were not properly handled, leading to erroneous clinical conclusions. As a general rule, the higher the incidence of CEs, the greater the overestimation produced when they are treated as “censored”. Furthermore, it has been shown that CE percentages > 10% require specific analyses that take them into consideration.14
During the follow-up of elderly patients with heart disease, such as those receiving biological prostheses, there is an elevated percentage of CEs. In our series, the patients’ mean age was 77 years and CEs were present in almost 40%, a value consistent with the reported percentages in other studies.5,8–12
With the analysis we used, robust data can be obtained in the presence of CEs. The results differed notably from those obtained with KM estimation (), underscoring the need to carry out a suitable analysis.
LimitationsThe timing of echocardiographic follow-up depended on each patient's treating cardiologist, and for this reason, there were several time intervals between the 2 echocardiographic examinations. Therefore, these data were interval-censored, and the midpoint imputation used, although robust, is an estimation. Nonetheless, most patients had at least 1 echocardiogram per year. We confirmed that patients who died had undergone echocardiography at some point during the year prior to their death (all except 19 patients). However, the 1-year time point is random and it is impossible to assure that during that year a patient will not develop asymptomatic SVD.
Elderly patients, who often have a small, calcified aortic annulus, are susceptible to developing PPM. This could have influenced the unusually high percentages of PPM and SVD in our series, and it should be taken into consideration when extrapolating the results.
CONCLUSIONSUsing a definition based on the transprosthetic pressure gradient increase, the incidence of SVD associated with the Mitroflow aortic valve prosthesis was higher than the values reported in other series, especially in smaller-sized valves (19 and 21mm) and in patients with severe PPM. The development of this complication accelerates exponentially starting from the fifth year following aortic valve replacement, making strict clinical and echocardiographic monitoring mandatory at that time. In the absence of effective early treatment, the patient's mortality risk undergoes a 4.5-fold increase once SVD has developed.
CONFLICTS OF INTERESTC. Morís is a proctor of Corevalve/Medtronic.
- –
The Mitroflow aortic prosthetic valve is specifically designed to improve the hemodynamic profile associated with biological prostheses. It has been implanted in tens of thousands of patients with small, calcified aortic annuli. However, the design of the prosthesis, together with the absence of anticalcification treatment, has cast doubts on the durability of the device.
- –
The development of SVD in this prosthetic valve accelerates exponentially starting from the fifth year postimplantation. At 8 years of follow-up, degeneration is seen in 1 of every 6 prostheses overall and in 1 of 5 smaller prostheses (19 or 21mm). At the time this late complication develops, the patient's risk of death undergoes a 4.5-fold increase.
The authors thank Lola García (echocardiographer) for her excellent work.