Hypertension is a major contributor to cardiovascular morbidity and mortality worldwide. For decades considerable effort has been dedicated to the prevention and treatment of this condition. Despite the development of various drug regimens and counseling on life style changes, a large percentage of patients do not achieve blood pressure (BP) reductions to the recommended values. Others are able to control BP with the use of several antihypertensive medications, but this implies substantial pharmaceutical expenditure and exposure to adverse effects, many of them severe.
The sympathetic nervous system (SNS) (together with the parasympathetic system) is a part of the autonomous nervous system,1 which regulates the involuntary functions of the body. These include BP regulation, a task in which the SNS plays a crucial role by acting through 2 types of successively connected neurons. The body of the first neuron (preganglionic neuron) is located in the thoracic and lumbar segments of the spinal cord (from T1 to T2), specifically in the intermediate/lateral horn. The body of the second sympathetic neuron (ganglionic neuron) is located outside the central nervous system, either in a bilateral paravertebral position in small interconnected autonomic ganglia (forming the paravertebral sympathetic chain) or in large distal abdominal sympathetic ganglia, known as prevertebral or preaortic ganglia (mainly the celiac, superior mesenteric, and inferior mesenteric ganglia). To perform their function, the ganglionic neurons connect with the target organ through postganglionic axons (Figure 1). Each preganglionic neuron synapses with multiple ganglionic neurons distributed at several levels, which explains the diffuse response throughout the body of a single sympathetic stimulus.
Diagram of the sympathetic nervous system connections. Two neurons, serially connected. In dotted blue, the preganglionic neuron located in the intermediate-lateral horn of the spinal cord. In red, the ganglionic neuron located in the paravertebral sympathetic chain or in the large, distal, prevertebral ganglia. The ganglionic neurons connect with the target organ where they perform their function. This figure is shown in full color only in the electronic version of the article.
The major neurotransmitters in the SNS include acetylcholine in the preganglionic neurons (cholinergic neurons) and noradreneline in the ganglionic neurons (adrenergic neurons), except in the suprarenal medulla, the main site of adrenaline secretion. These postganglionic neurotransmitters interact with sympathetic receptors (alpha and beta) in the various organs, where they produce their biological response. Renal juxtaglomerular cells are rich in B1 receptors, which stimulate renin secretion.
Activation of the SNS is essential in the pathogenesis of HT,2 particularly in young and middle-aged persons. SNS hyperstimulation favors the development and persistence of HT in healthy young individuals, which can lead to progressively more severe target organ injury. In this regard, nonselective surgical sympathectomy (splanchnicectomy) has been proven to decrease BP, but is associated with disabling adverse effects such as orthostatic hypotension, syncope, and sexual dysfunction.3
The renal sympathetic nerves within the SNS are key elements in HT. They participate in tubular sodium handling (sodium and water retention) and renin secretion, and they increase renal vascular resistance (decreasing the renal blood flow and glomerular filtration rate).4 As indicated, they are formed by the ganglionic nerves of the paravertebral sympathetic chain or the prevertebral ganglia anterior to the aorta. Renal innervation develops from these ganglia. In classic descriptions, when these nerves destined for the kidney (renal SNS) reach the renal artery (RA), they form a network known as the renal plexus. This network surrounds the main RA at the adventitia and passes through the hilum to enter and innervate the kidney. The proximal and medial segments have a larger number of periarterial nerves, and the ventral region has more nerves than the dorsal. Around 75% of these nerves are located at a distance of 4.28mm (range, <1 to> 10mm) from the RA lumen.5 The periadventitial distribution of the renal SNS, very close to the RA lumen, together with the role of these nerves in HT and the favorable outcome of nonselective surgical sympathectomy, laid the groundwork for the development of selective endovascular renal sympathectomy through a catheter placed in the arterial lumen to treat HT.
On this basis, numerous catheters were developed for endovascular renal denervation (RD). Most of them were catheters with 1 or various electrodes connected to a radiofrequency generator. By controlling the temperature and impedance using the specific algorithms from each manufacturer, it was possible to apply the appropriate amount of energy to damage the nerve at the contact point. After the excellent results of numerous studies performed with RD, the findings from the SYMPLICITY HTN-3 trial (randomized, sham-controlled, and primary outcome based on 24-hour Holter BP)6 were eagerly awaited. The RD group underwent 4 to 6 ablations in both main RA (left and right) using a monopolar catheter (Symplicity, Medtronic; Minneapolis, Minnesota, United States). Denervation began at the distal end of the RA and proceeded point-by-point to the proximal end in a helicoidal pattern. Theoretically, circumferential ablation of the renal plexus was ensured with the use of this spiral pattern. In addition, as the burn was not circular (ring pattern), it was believed that the incidence of renal stenosis would be reduced. However, this trial did not demonstrate that RD was superior to medical treatment, the technique suffered a major setback, and today it remains virtually forgotten. The question is: What went wrong?
The reasons why this trial failed, with its extensive background, have been investigated, and several possibilities have been identified. Among others, erroneous selection of the study population and poor treatment adherence have been cited, and most importantly, a general lack of knowledge regarding the macroscopic and microscopic renal neuroanatomy. Our group is working on anatomic study of the renal SNS,7,8 and we are currently focused on analyzing the causes that could explain the failure of this technique and how to optimize it from the anatomic viewpoint.
Much of the existing knowledge on renal neuroanatomy and that which was used to create the RD protocols, is based on small anatomic studies conducted at the beginning and middle of the 20th century. To our knowledge, the largest of these was performed in 9 adult cadavers.9 The most likely reason for the scarcity of renal SNS dissections in previous studies is that more than 40hours of work are needed to correctly expose the renal plexus from a block specimen. This lack of anatomic information resulted in an erroneous (from our viewpoint) RD protocol based on an overly simplified model, in which little importance was given to the denervation protocol. In short, the false expectation was created that RD would be effective regardless of what was done or where it was done: an “everything goes” idea.
Several anatomic causes could lead to failure of the RD procedure as it was understood in the past. First, the renal plexus is not a plexus; that is, small nerves all of equal size surrounding the adventitia of the RA as a network. In contrast, the renal SNS is composed of thick nerve bundles, arising from the axons of the sympathetic ganglionic neurons and tending to run along the superior, inferior, ventral, or dorsal RA. These nerve bundles often follow a spiral path and their course can change quadrants along the RA (Figure 2). Therefore, 2 strategies can be used to achieve effective RD (circumferential ablation in the 4 quadrants).
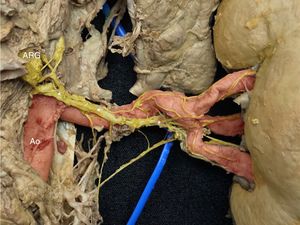
Right kidney, posterior view. The image shows a large nerve originating in the superior portion of the right posterior aorticorenal ganglion (ARG). Note how its position changes along the course of the main renal artery, passing from a superior to a posterior location (*). In red, the aorta (Ao) and the main and segmental renal arteries. In yellow, the renal sympathetic nerves. This figure is shown in full color only in the electronic version of the article.
In the first approach, using a unipolar catheter (which tends to produce damage in 90° of the arterial circumference), complete helicoidal ablation should be ensured by denervation of all points of the spiral, leaving none isolated. Furthermore, this should be done along a short segment of the artery. If it is not, because of the spiral course of the bundles, a nerve might cross to another quadrant between the ablation points and escape denervation. It also seems reasonable that the larger the number of random ablation points achieved, the greater the probability of adequately denervating the kidney.
The second RD strategy would be to perform a single circumferential RD in a ring configuration (not spiral) in a specific segment of the RA, which would avoid the problems occurring with the spiral configuration. This would theoretically increase the efficacy of the technique, but could lead to a higher incidence of renal stenosis at long term, as it would produce a 360° injury at a specific position in the artery.
In addition to these considerations, in a large percentage of kidneys, the main RA is not the one reaching the renal hilum. Before arriving at the hilum, the vessel tends to divide into 2 branches, the anterior segmental artery (running anterior to the renal pelvis and ureter) and the posterior segmental artery (posterior to the renal pelvis and ureter). When this prehilar division exists, some nerves commonly pass from the preaortic ganglia to 1 or both branches (thus, jumping over the main RA). This phenomenon is very important. It implies that in addition to denervating the main RA (which has been the radiofrequency recommendation to date), the renal artery branches would also have to be denervated in order to reach the nerves arriving distally. Another possibility would be to perform RD in the main RA, applying greater radiofrequency energy or using other energy sources (such as ultrasound), to access these renal nerves, which at this position are not as close to the main RA (Figure 3). As penetration is deeper with this last approach, it would have the potential drawback of producing unwanted damage to adjacent structures.
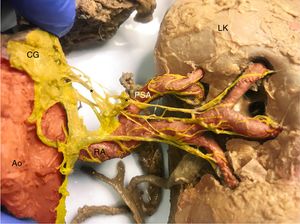
Anterior view of the left kidney (LK). A large nerve (*) originates in the celiac ganglion (CG). Note the nerve bundles uniting at the left posterior segmental artery (PSA). In red, the aorta (Ao), renal artery (RA), and segmental and subsegmental renal arteries. In yellow the renal sympathetic nerves. This figure is shown in full color only in the electronic version of the article.
In addition, renal vascularization is characterized by a large number of variations: a single RA, multiple RAs, arteries that reach the hilum (hilar arteries), those that penetrate the kidney outside the hilum (extrahilar arteries), and those that penetrate through the poles (polar arteries). Multiple renal arteries are relatively common (around 30% in the related studies). In this circumstance, the artery having the largest diameter is called the main artery and those with smaller diameters are termed accessories or supernumeraries. In patients with multiple renal arteries, we have found that sympathetic innervation is not only carried by the main RA; in most cases, the accessory arteries also carry nerves along their walls, regardless of their size (Figure 4). It seems reasonable that, to achieve an RD as complete as possible, it would be necessary to meticulously identify these multiple renal arteries and denervate all of them, not only the largest one.
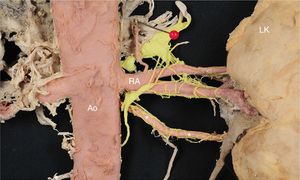
Anterior view of the left kidney (LK). Note the left inferior accessory polar artery (*), which carries numerous nerves along the vessel wall. In red, the aorta (Ao), renal artery (RA), and segmental and subsegmental renal arteries. In yellow, the renal sympathetic nerves. This figure is shown in full color only in the electronic version of the article.
The key question is, then: How should the endovascular RD procedure be focused to render it successful? In addition to taking into account the 3 points mentioned above and due to anatomic variability, it is important to carry out a study of the renal vasculature before the procedure and plan the strategy according to the findings. The idea that “anything you do is fine” is not useful in current endovascular RD.
In the same way that the interventional cardiologist is thoroughly familiar with the coronary artery anatomy, the person performing an RD procedure should have extensive knowledge of the renal vasculature, its variants, and the aortic branches around the RA. This information is essential to plan which segments to target and to determine the appropriate radiologic projections, the position of the catheter at each time point, which branches have been denervated and which still need to be, and which projections should be used to access them.
RENAL DENERVATION STUDIES, VARYING THE TECHNIQUEAfter the setback of Symplicity HTN-3, new proof-of-concept pilot studies were designed with maximum control of potential confounding variables to determine whether endovascular RD may actually be effective.
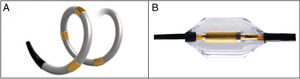
With regard to the procedure and ablation technique, 2 types of catheters have been used since HTN-3. The first is a spiral radiofrequency catheter equipped with 4 sequential electrodes to ensure that ablation will be circular and in a spiral course (Spyral, Medtronic; Minneapolis, Minnesota, United States). The second is an ultrasound catheter that achieves ring ablation in the same segment (Paradise, ReCor Medical; California, United States) (Figure 5). The ablation technique has also been modified. In RD with spiral radiofrequency, the segmental branches are treated in addition to the main RA and accessory branches. In ring RD with an ultrasound catheter, only the main RA and accessories are denervated. This procedure should only be performed by operators with extensive experience.
Information is available from 3 randomized, prospective, single-blinded, sham-controlled studies using these 2 devices, with attempts to control for all confounding variables. Two of them used RD with spiral radiofrequency provided by the Spyral catheter (SPYRAL HTN-OFF MED10 and SPYRAL HTN-ON MED11 pilot studies) and the third (RADIANCE-SOLO12) carried out ring RD using the Paradise ultrasound balloon catheter.
Once the safety of transcatheter RD had been demonstrated in previous clinical trials, an important novelty was that patients other than those with refractory HT were randomized to participate. Patients with mild or moderate essential HT despite receiving antihypertensive treatment (no more than 3 drugs in SPYRAL HTN-ON MED) were included, as well as those with mild/moderate HT who were not taking antihypertensive medication (SPYRAL HTN-OFF MED and RADIANCE-SOLO studies).
The results of these 3 studies are all very positive. First, an important aspect should be highlighted: none of the studies reported any adverse events or relevant complications related to the technique. This implies that the technique is safe, whether or not it may work in some patients. The focus of further effort in this line should be to find ways to identify which patients will respond to RD before the procedure. With regard to efficacy, these 3 studies demonstrated that RD significantly reduces BP in a persistent manner (Figure 6).
CONCLUSION AND FUTURE DIRECTIONSIn short, broad knowledge of the renal vasculature and neuroanatomy is essential to design and apply an appropriate procedure for selective renal sympathectomy. After the sharp drop in expectations regarding transcatheter RD and overcoming numerous problems, including strict control of confounding variables, SPYRAL ON MED, OFF MED, and RADIANCE-HTN SOLO provide the first evidence that RD is truly effective and safe. Now we can say the RD is effective therapy when properly performed and that it opens a new field to provide alternative BP control.
Nonetheless, many questions remain unanswered: How much denervation is needed to achieve an effect? Are there any markers to confirm the success of RD during the procedure? Which patients will respond well and which will not? Which patient populations can benefit from this therapy? What is the net clinical benefit? What is the long-term safety of the technique? How long do the effects of denervation last?
The currently ongoing RD studies (eg, the pivotal SPYRAL OFF-MED [NCT02439749] study, the extension of the pilot SPYRAL ON-MED [NCT02439775], RADIANCE-HTN TRIO and SOLO [NCT02649426] studies, and the RADIANCE-II [NCT03614260] study) will provide further data to answer all these questions and hopefully, many more.
CONFLICTS OF INTERESTA. García-Touchard is a consultant of Medtronic and Metavention Inc and has received grants from Medtronic for the study of the renal plexus.