We sought to determine the incidence of vascular complications in patients with chronic kidney disease undergoing primary angioplasty via the femoral route; we also evaluated the safety and efficacy of the use of vascular closure devices in this setting.
MethodsRegistry of 527 patients undergoing primary angioplasty via the femoral route from January 2003 to December 2008. Chronic kidney disease was defined as creatinine clearance less than 60mL/min. The primary endpoint was the presence of major vascular complications.
ResultsBaseline chronic kidney disease was observed in 166 (31.5%) patients. Patients with chronic kidney disease experienced higher rates of major vascular complications compared to those without worsening of renal function (8.4% vs 4.2%; P=.045), especially those requiring transfusion (6.6% vs 1.9%; P=.006). Among patients with chronic kidney disease, 129 (77.7%) received a vascular closure device and manual compression was used in 37 patients (22.3%). The risk of major vascular complications was significantly lower with vascular closure device use compared to manual compression (4.7% vs 21.6%; P=.003). Multivariable logistic regression analysis showed that the use of a vascular closure device was independently associated with a decreased risk of major vascular complications in patients with chronic kidney disease undergoing primary angioplasty (odds ratio=0.11; 95% confidence interval, 0.03-0.41; P=.001).
ConclusionsPatients with chronic kidney disease undergoing primary angioplasty via the femoral route experience higher rates of major vascular complications. The use of vascular closure devices in this group of patients is safe and is associated with lower rates of major vascular complications compared to manual compression.
Keywords
.
INTRODUCTIONChronic kidney disease (CKD) is independently associated with increased cardiovascular morbidity, including the increased risk of acute myocardial infarction.1 Given the increased prevalence of CKD in the western world, it is unsurprising that patients with impaired kidney function are an increasingly important group among patients admitted to emergency cardiac catheterization laboratories for primary angioplasty (PA) in the treatment of ST-segment elevation acute coronary syndrome (STEACS). Impaired kidney function has been associated with an increased risk of bleeding and complications related to vascular access after percutaneous coronary intervention (PCI).2 However, in the specific setting of PA, where the risk is particularly high due to the need for intensive anticoagulation and antiplatelet therapy, the actual incidence of complications related to vascular access in the group of patients with CKD remains unknown.
Different strategies have been developed to reduce bleeding complications after PCI, such as the use of new anticoagulants like bivalirudin, or the preferential use of the radial access route.3, 4 The use of vascular closure devices (VCDs) for this purpose remains controversial.5 Moreover, there is little information on the safety of these devices in patients at high risk of bleeding, such as patients with CKD or those undergoing PA, as these patients have been excluded from most studies that have evaluated the use of VCDs.6 In the specific case of patients with CKD, recent studies have reported an increased risk of vascular complications with the use of VCDs.7
The aim of our study was to analyze the incidence of vascular complications in patients with CKD treated with PA via the femoral artery compared to patients without impaired kidney function and to determine the safety and efficacy of VCDs in patients with CKD treated with PA.
METHODS Background and Study PopulationThe Servicio Galego de Saúde (SERGAS; Health Service of Galicia) has launched the PROGALIAM program to ensure access to PA for most of the Galician population. The details of this program have been described previously.8, 9 Briefly, patients with STEACS who are admitted to hospital and are candidates for interventional reperfusion, as defined in the clinical practice guidelines, are treated with urgent PCI. Patients who are first admitted to a noninterventionist hospital are quickly transferred to a hospital with a cardiac catheterization laboratory via the 061 emergency ambulances to receive the same treatment. All patients who presented with typical anginal pain of more than 30min duration with ST elevation ≥1mm in 2 or more contiguous leads (or with reciprocal depression ≥1mm in leads V1 or V2) or left bundle branch block and were eligible for PA within the first 12h after the onset of symptoms were included in the study if the procedure was performed using the femoral route. Patients who died during the procedure were excluded from the study as well as those who required intraaortic balloon counterpulsation via the same route by which the procedure was performed. Information on their clinical characteristics, cardiovascular risk factors and previous treatments was collected directly from the patient or, if necessary, from medical records.
Primary Angioplasty ProtocolInterventional cardiologists with proven experience performed all the PA according to the clinical practice guidelines. Femoral artery cannulation was performed using the Seldinger technique after the anatomical landmarks were identified. The most frequently used introducers were 6 Fr; only in the case of more complicated interventions were 7 Fr introducers used. All patients received 250mg of acetylsalicylic acid at the time of diagnosis. A 300-mg loading dose of clopidogrel was administered in the emergency department or during transport by ambulance. If a loading dose had not been received, this was done following angioplasty and before the patient left the cardiac catheterization laboratory. The use of the glycoprotein IIb/IIIa inhibitor abciximab (ReoPro®; 0.25mg/kg loading dose followed by 0.125μg/kg/min infusion over 12h) was strongly recommended in the protocol, although its use was left to the discretion of the physician who initially treated the patient. During catheterization, an intravenous dose of 60 IU/kg unfractionated heparin with abciximab or 100 IU/kg without abciximab was administered. After the procedure a maintenance dose of 75mg/day of clopidogrel for 1 month was recommended in the case of bare-metal stent implantation or for 12 months in the case of drug-eluting stents.
Occlusion ProtocolIn each case, the interventionist chose the femoral artery closure technique. Without exception, when the use of a VCD was considered, femoral angiography was performed prior to implantation. Generally, the device was not used if the puncture site was not located in the common femoral artery, the artery was of small caliber (less than 5mm), or there was severe peripheral artery disease. A choice of device was available to the operator: Angio-Seal® (St. Jude Medical; St. Paul, Minnesota, United States), StarClose® (Abbott Laboratories; Abbott Park, Illinois, United States) and Perclose A-T® (Abbott Laboratories, Illinois, United States). Device implantation was performed in the cardiac catheterization laboratory directly after the procedure and according to the manufacturer's instructions. In cases where manual compression was performed, the introducer was removed in the cardiac catheterization laboratory at the end of the procedure and manual pressure was applied to the puncture site for 15min to 20min. If needed, a mechanical compression device was used to achieve total occlusion. Subsequently, an inguinal compression bandage was applied for 8h. According to both the protocol and the clinical practice guidelines, the patients in both groups were not permitted to walk until 12h to 24h after the PA procedure.
Definitions and EventsCKD was defined as creatine <60/mL/min as calculated by the Cockcroft-Gault formula: (140–age)×(weight) (×0.85 if female)/(72×serum creatinine) during baseline blood tests at admission. This formula has been validated and accurately predicts the glomerular filtration rate.10 To identify the spectrum of kidney disease in the study population and its impact on major vascular complications (MVC), the patients with CKD were divided into 2 groups if they presented an estimated creatinine clearance of 30mL/min to 60mL/min or <30mL/min (severe CKD). All patients were examined at admission to assess the presence of complications related to vascular access. Systematic blood analysis was conducted both at admission and between 24h and 72h after the procedure. We defined MVC as the composite event of all complications related to vascular access that were fatal or required surgical or endovascular repair or transfusion, or those associated with a fall in hemoglobin ≥3g/dL. This definition is based on that provided by the Organization to Assess Strategies in Acute Ischemic Syndromes.11 We also assessed the presence of minor vascular complications, including hematoma, arteriovenous fistula, pseudoaneurysm, bleeding not fulfilling criteria for MVC, and infection at the vascular access site. A VCD failure was defined as aborted implantation or ineffective implantation due to poor occlusion requiring manual or mechanical compression. The analysis was performed with intention-to-treat; thus, in the case of device failure, the patient was included in the VCD group. Thirty-day mortality was also assessed.
Statistical AnalysisThe results are presented as mean (1 standard deviation) for normally distributed continuous variables, as median [interquartile range] for non-normally distributed continuous variables, and as percentages for categorical variables. Categorical variables were compared using the χ2 test or Fisher's exact test. Quantitative variables were analyzed using the t-test or Mann-Whitney test, depending on whether the distribution was normal or not. To assess the independent effect of CKD on the incidence of MVC, we constructed a logistic regression model including the variables independently associated with the presence of MVC in our sample (sex and use of VCDs) and other variables that, based on previous studies, clinical experience or asymmetric distribution between groups, were considered potential confounders (age, body surface area, diabetes mellitus, peripheral vascular disease, shock and use of abciximab). The variables were introduced in the model in blocks. Similarly, we constructed a logistic regression model including the same variables to determine the independent effect of using VCDs on MVC in the group of patients with CKD. A P-value of <.05 was used as a cutoff for statistical significance. All statistical analysis was performed using the SPSS 15.0 statistical package for Windows (SPSS, Chicago, Illinois, United States).
RESULTSBetween January 1, 2003 and December 31, 2008, 527 patients who fulfilled the inclusion criteria were treated with PA via the femoral artery (32% of all PAs performed during this period). In total, 166 (31.5%) had CKD and of these 23 (13.9%) had a creatinine clearance of <30mL/min. Of those with CKD, 129 (77.7%) received a VCD and in 37 (22.3%) manual compression was applied. Of the patients with CKD who received a VCD, an Angio-Seal® device was used in 86 (66.7%), a Perclose A-T® device in 25 (19.4%), and a StarClose® device in 18 (13.9%).
Vascular Complications in Patients With Chronic Kidney Disease Treated by Primary Angioplasty Via the Femoral RouteCompared to patients without impaired kidney function, patients with CKD at baseline were significantly older, had a higher prevalence of diabetes mellitus, hypertension, and peripheral artery disease, and were less often treated with abciximab. It should be noted that VCDs were used less frequently in the group of patients with CKD. Table 1 shows the clinical characteristics and procedures applied in the total study population.
Table 1. Baseline and Procedure Characteristics of the Total Study Population.
| Kidney disease (n=166) | Control Group (n=361) | P | |
| Age, years | 72.3±9.9 | 60.2±11.7 | <.001 |
| Males | 121 (72.9) | 285 (78.9) | .125 |
| Body surface area (m2) | 1.8±0.2 | 1.8±0.3 | .708 |
| Hypertension | 59 (35.5) | 78 (21.6) | .001 |
| Dyslipidemia | 27 (16.3) | 66 (18.3) | .573 |
| Smokers | 12 (7.2) | 64 (17.7) | .001 |
| Diabetes mellitus | 31 (18.7) | 33 (9.1) | .002 |
| Peripheral artery disease | 16 (9.6) | 13 (3.6) | .005 |
| History of AMI | 5 (3) | 12 (3.3) | .851 |
| History of coronary surgery | 2 (0.6) | 2 (1.2) | .594 |
| Anterior infarction | 71 (43) | 163 (45.2) | .650 |
| Cardiogenic shock | 14 (8.4) | 9 (2.5) | .002 |
| Creatinine clearance (mL/min) | 43.7±11.1 | 91.6±28.3 | <.001 |
| Procedure characteristics | |||
| Abciximab | 96 (57.8) | 258 (71.5) | .002 |
| Clopidogrel | 154 (94.5) | 344 (96.1) | .407 |
| Symptoms-to-reperfusion time (min) | 237 [177-378] | 220 [160-322] | .114 |
| Door-to-balloon time (min) | 122 [86-173] | 111 [80-150] | .031 |
| Angiographic success | 160 (97) | 355 (98.3) | .333 |
| 7 Fr introducer | 5 (3) | 10 (2.8) | .877 |
| Vascular closure devices | 129 (77.7) | 307 (85) | .039 |
AMI, acute myocardial infarction.
Data are expressed as mean±standard deviation for normally distributed variables, as medians [interquartile range] for non-normally distributed variables and as no. (%) for categorical variables.
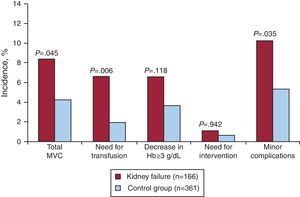
Patients with CKD had a higher incidence of MVCs than patients without impaired kidney function (8.4% vs 4.2%, P=.045). Among those with MVC, there was a greater incidence of femoral bleeding that required transfusion among patients with CKD (6.6% vs 1.9%, P=.006). Similarly, patients with CKD had a higher incidence of minor vascular complications (10.2% vs 5.3%, P=.035). On the other hand, in the study population, the level of impaired kidney function was also associated with an increased incidence of MVC (4.2% in patients without CKD vs 7% in patients with a creatinine clearance 30mL/min to 60mL/min vs 17.4% in patients with severe CKD, P=0.017). Thirty-day mortality was significantly higher in patients with CKD than in patients without impaired kidney function (11.4% vs 1.7%, P<.001). Table 2 and Figure 1 show the events in the total study population.
Table 2. Summary of Events in the Total Study Population.
| Chronic kidney disease (n=166) | Control group (n=361) | P | |
| MVC | 14 (8.4) | 15 (4.2) | .045 |
| Transfusion | 11 (6.6) | 7 (1.9) | .006 |
| Decrease in hemoglobin ≥3 g/dL | 11 (6.6) | 13 (3.6) | .118 |
| Need for intervention | 4 (1.1) | 1 (0.6) | .942 |
| Retroperitoneal hemorrhage | 2 (1.2) | 4 (1.1) | .923 |
| Minor vascular complications | 17 (10.2) | 19 (5.3) | .035 |
| Pseudoaneurysm | 1 (0.6) | 1 (0.3) | .531 |
| Arteriovenous fistula | 2 (1.2) | 2 (0.6) | .594 |
| Minor bleeding/hematoma | 13 (7.8) | 16 (4.4) | .112 |
| Infection | 1 (0.6) | 0 (0) | .315 |
| Death at 30 days | |||
| Total | 19 (11.4) | 6 (1.7) | <.001 |
| Vascular | 1 (0.6) | 1 (0.3) | .531 |
MVC, major vascular complications.
Data are expressed as no. (%).
Figure 1. Bar graph showing the incidence of vascular complications in the total population in relation to chronic kidney disease. Hb, hemoglobin; MVC, major vascular complications.
In the regression model adjusted for potential confounders, CKD was associated with an increased risk of MVC, although this result was not statistically significant (odds ratio [OR]=2; 95% confidence interval [95%CI], 0.92-4.43; P=.081). There was an independent association between severe CKD and MVC (OR=3.4;95%CI, 1.1-11.7; P=.043). In this model, in addition to severe CKD, female sex (OR=3.8;95%CI, 1.7-8.3; P=.001) and the use of a VCD (OR=0.4; 95%CI, 0.2 to 0.9; P=.029) were independently associated with MVC.
Safety and Efficacy of Vascular Closure Devices in Patients With Chronic Kidney Disease Treated With Primary AngioplastyIn the group of patients with CKD, there were no significant differences in baseline characteristics and procedures between patients who received a VCD and those who underwent manual compression (Table 3).
Table 3. Baseline and Procedure Characteristics of the Patients With Chronic Kidney Disease.
| Vascular closure devices (n=129) | Manual compression (n=37) | P | |
| Age, years | 72.8±9.6 | 70.9±10.7 | .322 |
| Males | 95 (73.6) | 26 (70.3) | .684 |
| Body surface area (m2) | 1.8±0.3 | 1.8±0.1 | .953 |
| Hypertension | 44 (34.1) | 15 (40.5) | .471 |
| Dyslipidemia | 22 (17.1) | 5 (13.5) | .607 |
| Smokers | 12 (9.3) | 0 | .070 |
| Diabetes mellitus | 22 (17.1) | 9 (24.3) | .317 |
| Peripheral artery disease | 11 (8.5) | 5 (13.5) | .355 |
| History of AMI | 3 (2.3) | 2 (5.4) | .312 |
| History of coronary surgery | 2 (1.6) | 0 | .987 |
| Anterior infarction | 57 (44.5) | 14 (37.8) | .469 |
| Cardiogenic shock | 9 (7) | 5 (13.5) | .310 |
| Creatinine clearance (mL/min) | 42.7±13.7 | 47±10.3 | .085 |
| Procedure characteristics | |||
| Abciximab | 74 (57.4) | 22 (59.5) | .820 |
| Clopidogrel | 121 (96) | 33 (89.2) | .119 |
| Symptoms-reperfusion time (min) | 254 [190-389] | 212 [153-294] | .131 |
| Door-to-balloon time (min) | 126 [87-189] | 120 [78-157] | .248 |
| 7 Fr introducer | 4 (3.1) | 1 (2.7) | .899 |
| Angiographic success | 126 (98.4) | 34 (91.9) | .075 |
AMI, acute myocardial infarction.
Data are expressed as mean±standard deviation for normally distributed variables, as medians [interquartile range] for non-normally distributed variables and as no. (%) for categorical variables.
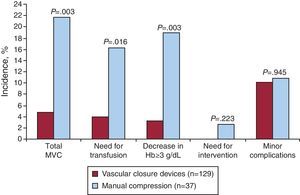
Among patients with CKD treated with PA via the femoral artery, the use of VCDs was associated with a lower incidence of MVC than with the use of manual compression (4.7% vs 1.6%, P=.003). The use of VCD in the group of patients with CKD was associated with a decreased need for transfusion (3.9% vs 16.2%, P=.016) and a lower incidence of bleeding associated with a fall in hemoglobin ≥3g/dL (3.1% vs 18.9%, P=.003). There were no differences between groups in minor vascular complications in relation to individual components or in combination. Neither were there differences between groups in 30-day mortality. Failure of the VCD in patients with CKD occurred in 8 (6.2%) patients. Among patients with CKD who were implanted with a VCD, device failure was associated with greater incidence of MVC than when implantation was successful (25% vs 3.3%, P=.036). In Table 4 and Figure 2 summary of events in the group of patients with CKD.
Table 4. Summary of Events in the Group of Patients With Chronic Kidney Disease.
| Vascular closure devices (n=129) | Manual compression (n=37) | P | |
| MVC | 6 (4.7) | 8 (21.6) | .003 |
| Transfusion | 5 (3.9) | 6 (16.2) | .016 |
| Decrease in hemoglobin≥3 g/dL | 4 (3.1) | 7 (18.9) | .003 |
| Need for intervention | 0 | 1 (2.7) | .223 |
| Retroperitoneal hemorrhage | 1 (0.8) | 1 (2.7) | .397 |
| Minor vascular complications | 13 (10.1) | 4 (10.8) | .945 |
| Pseudoaneurysm | 0 | 1 (2.7) | .223 |
| Arteriovenous fistula | 2 (1.6) | 0 | .603 |
| Minor bleeding/hematoma | 10 (7.8) | 3 (8.1) | .944 |
| Infection | 1 (0.8) | 0 | .777 |
| Death at 30 days | |||
| Total | 13 (10.1) | 6 (16.2) | .378 |
| Vascular | 0 | 1 (2.7) | .223 |
MVC, major vascular complications.
Data are expressed as no. (%).
Figure 2. Bar graph showing the incidence of vascular complications in the group of patients with chronic kidney disease in relation to the use of vascular closure devices. Hb, hemoglobin; MVC, major vascular complications.
In the logistic regression analysis adjusted for potential confounders, the use of VCDs in patients with CKD treated with PA was independently associated with a decreased risk of MVC (OR=0.11; 95%CI, 0.03-0.41, P=.001). In addition to the use of VCDs, only body surface area was independently associated with MVC in patients with CKD (OR=0.02; 95%CI, 0.01-0.7; P=.033)
DISCUSSIONThis is the first study to assess the incidence of complications related to vascular access in patients with CKD treated with PA via the femoral artery in the setting of STEACS, as well as the safety and efficacy of VCDs in this group of patients.
Vascular Complications in the Group of Patients With Chronic Kidney Disease Treated With Primary Angioplasty Via the Femoral RouteSeveral studies have found a strong association between impaired kidney function and bleeding complications following PCI and in the setting of PA itself.12, 13 However, the specific incidence of bleeding complications related to vascular access in patients with CKD treated with PA via the femoral artery has not been previously published. Our results show a higher incidence of femoral vascular complications in patients with CKD than in patients without impaired kidney function; the risk of MVC is also higher the greater the deterioration in renal function. It should be highlighted that there was an increased risk of femoral bleeding requiring blood transfusion in patients with CKD.
Several mechanisms may explain the increase risk of bleeding complications in patients with CKD following PCI. First, there is a high prevalence of associated comorbidities in this population that may partly explain the increased risk of bleeding.13 Second, it has been suggested that impaired kidney function itself has a direct effect on the risk of bleeding, mediated by the endothelial and platelet dysfunction that occurs in uremia.14 Thus, in the present study, patients with CKD were older and had a higher prevalence of diabetes mellitus and peripheral artery disease; after adjustment, the analysis showed that severe CKD alone was independently associated with MVC.
In the study population, and consistent with the results reported in previous studies, CKD was associated with increased mortality following PA.13 It remains to be determined to what degree this increase in mortality is explained by the greater incidence of bleeding complications in patients with CKD.
Impact of the Use of Vascular Closure Devices in the Group of Patients With Chronic Kidney Disease Treated With Primary AngioplastyThe use of VCDs for the prevention of bleeding complications following PCI remains controversial.15 Up to the present, several studies have shown a decrease in vascular complications with the use of VCDs.16 In contrast, other studies have noted an increase in local complications with the use of VCDs compared to manual compression.17 Finally, the majority of randomized studies have reported no effect.6 A recent metaanalysis,5 which assessed 31 clinical trials that included 7528 patients undergoing diagnostic and therapeutic coronary interventions, showed that although the use of VCD was associated with shorter occlusion times, this did not significantly decrease local bleeding complications compared to manual compression.
However, certain aspects should be kept in mind when interpreting these results. First, the available information on the use of VCDs in patients at increased risk of bleeding, such as those undergoing an emergency procedure or with severely impaired kidney function, is very scarce given that these patients have been routinely excluded from studies assessing VCD use. In this sense, the ACUITY study18 showed that in patients with acute coronary syndrome without ST elevation undergoing early PCI the use of VCDs was associated with a significant decrease in major bleeding complications. In the setting of STEACS treated with PA via the femoral route, the results obtained from our total study population showed that the use of VCDs was independently associated with a lower incidence of MVC compared to manual compression.19
There is even less information available on the safety and efficacy of using VCDs in patients with CKD. In a recent study, Aziz et al.7 reported a high risk of vascular complications with the use of VCDs in patients with CKD undergoing PCI. However, any conclusions that can be drawn from this study are limited due to the lack of a manual compression control group. The present study is the first to specifically assess the use of VCDs in patients with CKD in a setting in which there is a particularly high risk of bleeding, such as that of PA. Our results show that the use of VCDs in this population is safe and is associated with a low incidence of vascular complications, lower than in the manual compression group and very similar to that of patients without CKD. These results are consistent with what has been reported for the total study population.19
The second point to note is that the results of clinical trials do not appear to reflect actual clinical practice, in which strict occlusion protocols may not always be established. Thus, consistent with our results, several recently published registries that have assessed the use of VCDs in clinical practice have described favorable outcomes in the prevention of bleeding complications.3, 16, 20, 21 In a registry of more than 1.5 million patients who underwent PCI, Marso et al.3 found that the use of VCDs was associated with a lower incidence of major bleeding complications, particularly in patients at higher risk of bleeding, including patients undergoing urgent procedures or those with CKD. Paradoxically, the register shows that patients at a higher risk of bleeding receive a VCD less frequently. Similarly, in our study population, the use of VCDs was lower among patients with CKD compared to patients without impaired kidney function.
Finally, in a recent review of strategies for the prevention of bleeding complications following PCI, Dauerman et al.22 concluded that there is now sufficient evidence to support the use of VCDs for this purpose. This conclusion appears to be very solid, particularly regarding patients at a higher risk of bleeding. In this line, the results of our study have the advantage of being directly applicable in clinical practice in a setting in which conducting randomized trials is difficult, and thus it seems reasonable to consider the use of VCDs in patients with CKD treated with PA via the femoral artery.
LimitationsThis study has several limitations that should be considered when interpreting the results. First, the nonrandomized observational design of the study could have introduced a selection bias that remained uncontrolled during the statistical analysis. In this regard, the number of patients with CKD who received a VCD was significantly different to the number of those receiving manual compression because the technique used to close the femoral artery was left to the discretion of the interventional cardiologist. Thus, it is possible that patients selected for manual compression were a group at increased risk of bleeding, with worse anatomical distribution or unfavorable femoral puncture sites. Second, our cardiac catheterization laboratory does not systematically measure activated clotting time, thus the relationship between the level of anticoagulation administration and the occurrence of bleeding complications could not be assessed.
CONCLUSIONSPatients with CKD treated with PA via the femoral artery in the setting of STEACS are at greater risk of MVC than patients without impaired kidney function. The use of VCDs in patients with CKD undergoing PA is safe and is associated with a decrease in MVC compared to manual compression.
FundingThis work was partly supported by the RECAVA Cardiovascular Network from the Ministry of Science and Innovation, Instituto de Salud Carlos III, Madrid, Spain.
Conflicts of InterestNone declared.
Acknowledgement
Dr. Rodrigo Estévez-Loureiro has been awarded the “Rio Hortega” research grant from the Ministry of Science and Innovation, Instituto de Salud Carlos III, Madrid, Spain.
Received 16 September 2011
Accepted 28 October 2011
Corresponding author: Servicio de Cardiología, Complejo Hospitalario Universitario de A Coruña, As Xubias 84, 15006 A Coruña, Spain. Oscar.Prada.Delgado@sergas.es