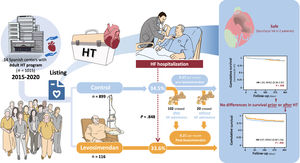
Repetitive ambulatory doses of levosimendan are an option as a bridge to heart transplantation (HT), but evidence regarding the safety and efficacy of this treatment is scarce. The objective of the LEVO-T Registry is to describe the profile of patients on the HT list receiving levosimendan, prescription patterns, and clinical outcomes compared with patients not on levosimendan.
MethodsWe retrospectively reviewed all patients listed for elective HT from 2015 to 2020 from 14 centers in Spain.
ResultsA total of 1015 consecutive patients were included, of whom 238 patients (23.4%) received levosimendan. Patients treated with levosimendan had more heart failure (HF) admissions in the previous year and a worse clinical profile. The most frequent prescription pattern were fixed doses triggered by the patients’ clinical needs. Nonfatal ventricular arrhythmias occurred in 2 patients (0.8%). No differences in HF hospitalizations were found between patients who started levosimendan in the first 30 days after listing and those who did not (33.6% vs 34.5%; P=.848). Among those who did not, 102 patients (32.9%) crossed over to levosimendan after an HF admission. These patients had a rate of 0.57 HF admissions per month before starting levosimendan and 0.21 afterwards. Propensity score matching analysis showed no differences in survival at 1 year after listing between patients receiving levosimendan and those who did not (HR, 1.03; 95%CI, 0.36-2.97; P=.958) or in survival after HT (HR, 0.97; 95%CI, 0.60-1.56; P=.958).
ConclusionsRepetitive levosimendan in an ambulatory setting as a bridge to heart transplantation is commonly used, is safe, and may reduce HF hospitalizations.
Keywords
Heart failure (HF) is a leading cause of morbidity and mortality worldwide, with an estimated prevalence of 1% to 2% of adults in developed countries.1,2 Current therapeutic options have significantly improved symptoms and prognosis, but heart transplantation (HT) remains the last resort for patients with advanced heart failure (AHF).3–5 However, HT is restricted due to the limited number of donors, leading to varying waiting times on the transplant list, which may be longer in large recipients or those with blood type 0.6 Patients on the waiting list are especially vulnerable as they have a high risk of HF admission and cardiogenic shock, which can jeopardize their survival, eligibility, or outcomes after HT.7–9 Therefore the option to bridge to HT with mechanical circulatory support or inotropes may be needed in high-risk recipients.5,10,11
Levosimendan is an intravenous inodilator drug that enhances the sensitivity of sarcomeres to calcium, thereby increasing myocardial contractility without elevating intracellular calcium levels, unlike other inotropes. In addition, levosimendan acts as a vasodilator by activation of adenosine triphosphate (ATP)-dependent potassium channels in vascular muscle cells.12 The Pharmacological features of levosimendan result in the presence of a long-lasting active metabolite in plasma after infusion, allowing repetitive ambulatory cycles in chronic AHF patients.13 Previous studies have shown that this treatment modality with levosimendan can decrease N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels and may reduce mortality and rehospitalization, but the evidence is inconsistent.14–17
In some countries, levosimendan is frequently used in AHF patients. However, limited information is available on the characteristics of patients treated with levosimendan, its mode of administration, and its safety. Our main objective was to describe the clinical profile of patients receiving levosimendan in real-world clinical practice among patients on the elective waiting list for HT. We also aimed to examine the prescription patterns and safety of this treatment in this population. Our secondary aims were to describe clinical events, including mortality, hospitalizations, and other relevant outcomes, and to compare these events with those in a control group of patients on the elective waiting list for HT who did not receive levosimendan.
METHODSStudy populationAll Spanish centers with an adult HT program (N=16) were invited to participate in the LEVO-T registry. Inclusion criteria encompassed all patients older than 18 years who were included on the elective waiting list between January 1, 2015 and September 1, 2020. Patients were retrospectively reviewed and followed up until June 2021. Those that received more than 1 dose of ambulatory levosimendan while on the waiting list and before HT were included in the treatment group, while patients not requiring levosimendan were included in the control group. We excluded patients with left ventricular assist devices at the time of inclusion on the waiting list. The decision to administer levosimendan treatment was made by each individual center based on their local criteria, which were established according to prior clinical experience. The Spanish Transplant Organization does not prioritize adult patients on inotropes, who therefore remain on the elective HT list.
The study was approved by the Hospital Universitari de Bellvitge ethics committee, and conformed to the principles of the Helsinki Declaration and with the International Society for Heart and Lung Transplantation (ISHLT) ethics statement. All patients signed an informed consent form for prospective collection of their anonymized data as part of their inclusion in the Spanish Heart Transplant Registry. The authors from each participating center guarantee the integrity of the data. This study was designed in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.
Data acquisitionData were retrieved by each center from the Spanish Heart Transplant Registry and electronic medical records (see appendix 2). The baseline evaluation was conducted at the time of inclusion on the HT waiting list. Variables included demographic characteristics, HF symptoms, comorbidities, previous HF admissions, medical treatment, and devices. Complementary tests included laboratory tests, echocardiography, and right heart catheterization before inclusion. Among patients receiving levosimendan, additional data were gathered at the time of the first administration and included dose, infusion speed, number of doses, vital signs, laboratory tests, echocardiographic parameters, and right heart catheterization data. Events collected during follow-up included the following: ventricular arrhythmias requiring admission to the emergency department or implantable cardioverter defibrillator therapies for ventricular arrhythmias; HF admissions defined as hospitalization for HF for> 24hours; need for mechanical circulatory support; inclusion on the urgent or emergent waiting list, HT, or death. Among patients who received a HT, additional information including laboratory tests and echocardiographic parameters before HT were collected, as well as data on primary graft dysfunction according to the ISHLT consensus definition,18 need for extracorporeal membrane oxygenation (ECMO), and survival after HT. To ensure the quality of the data, several criteria were established: a) data could only be included if they were within a range of reasonable values for each quantitative variable, previously defined by the research team; b) the dates of the different periods analyzed had to be correlative; c) before the statistical analysis, a search was made for missing and anomalous data and researchers were asked to make a review.
Statistical analysisNormally distributed continuous variables are expressed as mean±standard deviation (SD) while nonnormally distributed variables are expressed as median [interquartile range]. Continuous variables are presented as number and percentage for categorical variables. The Kolmogorov-Smirnov test was used to assess normal distribution in continuous variables. The Student t-test and Mann-Whitney U test were used to compare variables with and without normal distribution, respectively. The chi-square test was used to compare categorical variables. The Student t-test for paired samples was used to compare changes in patients’ characteristics during follow-up.
The Kaplan-Meier method was used to describe the survival of patients on the waiting list and after HT. Follow-up was censored at the time of HT or exclusion from the waiting list due to ineligibility. Differences between groups were analyzed with Cox regression models. Since differences were found between the levosimendan group and the control group, we carried out a pairing with the propensity score matching technique to minimize these differences. We adjusted the following covariates (table 1 of the supplementary data): age, sex, ischemic etiology, and the following parameters at the time of inclusion on the waiting list: weight, body mass index, New York Heart Association (NYHA) IV functional class, number of previous HF admissions, implantable cardioverter defibrillator carrier, CRT carrier, use of neurohormonal treatment and diuretics, creatinine values, left ventricular ejection fraction, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, cardiac output, and pulmonary vascular resistance. To perform the pairing, a nonparsimonious logistic regression analysis was first performed, with the dependent variable being “receiving levosimendan” and the independent variables being those previously mentioned. Then, 1:1 pairing was performed and the condition to generate each pair was that the difference in the probabilities was less than 0.024 (20% of the SD of the probabilities, which was 0.12). We then checked that the covariates were balanced between the 2 groups in 2 ways: checking the absence of significant differences (P> .05) and calculating the standardized difference between the 2 groups for each covariate. Small values (≤ 10%) were considered to support the equilibrium assumption in both groups.
The rate of HF admissions was compared between patients who started levosimendan in the first 30 days after inclusion with those who did not with the Student t-test for paired samples. Additionally, a subanalysis of survival and adverse effects was performed between patients with flexible and other dose patterns.
The statistical analysis was performed using SPSS software version 21 (IBM Corp, United States).
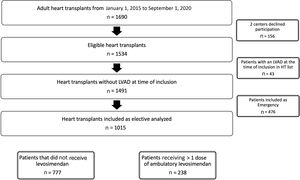
RESULTSInformation from 1015 consecutive patients included on the elective HT waiting list was provided by 14 out of 16 (87.5%) active centers with an adult HT program in Spain (figure 1). Detailed information on participating centers and the number of patients provided by each center are available in tables 2 and 3 of the supplementary data. Overall, 238 patients (23.4%) received> 1 dose of ambulatory levosimendan, while 777 patients (76.6%) did not require levosimendan, comprising the control group.
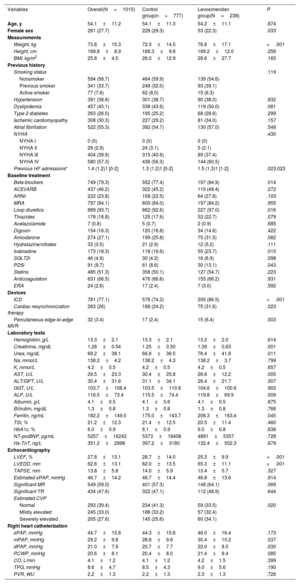
Table 1 displays the main baseline characteristics of the cohort at the time of HT listing. The mean age was 54.1±11.2 years and 27.7% were female. The underlying cause of HF was ischemic cardiomyopathy in 308 (30.3%) of patients, 522 (55.3%) had concomitant atrial fibrillation, and most patients (n=580, 57.3%) were in NYHA class IV. Overall, the patients were on optimal medical treatment for HF except for low prescription of sodium-glucose cotransporter 2 inhibitors during the study period (2015-2020). Mean NT-proBNP levels were 5257±16 242 pg/mL, and mean left ventricular ejection fraction was 27.8±13.1%.
Baseline characteristics of the cohort at the time of HT listing
| Variables | Overall(N=1015) | Control group(n=777) | Levosimendan group(N=238) | P |
|---|---|---|---|---|
| Age, y | 54.1±11.2 | 54.1±11.3 | 54.2±11.1 | .874 |
| Female sex | 281 (27.7) | 228 (29.3) | 53 (22.3) | .033 |
| Measurements | ||||
| Weight, kg | 73.8±15.3 | 72.3±14.5 | 76.8±17.1 | <.001 |
| Height, cm | 168.8±8.9 | 168.3±9.8 | 169.2±12.0 | .256 |
| BMI, kg/m2 | 25.8±4.5 | 26.0±12.9 | 28.6±27.7 | .165 |
| Previous history | ||||
| Smoking status | .119 | |||
| Nonsmoker | 594 (58.7) | 464 (59.9) | 139 (54.6) | |
| Previous smoker | 341 (33.7) | 248 (32.0) | 93 (39.1) | |
| Active smoker | 77 (7.6) | 62 (8.0) | 15 (6.3) | |
| Hypertension | 391 (38.6) | 301 (38.7) | 90 (38.0) | .832 |
| Dyslipidemia | 457 (45.1) | 338 (43.6) | 119 (50.0) | .081 |
| Type 2 diabetes | 263 (26.0) | 195 (25.2) | 68 (28.6) | .299 |
| Ischemic cardiomyopathy | 308 (30.3) | 227 (29.2) | 81 (34.0) | .157 |
| Atrial fibrillation | 522 (55.3) | 392 (54.7) | 130 (57.0) | .548 |
| NYHA | .430 | |||
| NYHA I | 0 (0) | 0 (0) | 0 (0) | |
| NYHA II | 29 (2.9) | 24 (3.1) | 5 (2.1) | |
| NYHA III | 404 (39.9) | 315 (40.6) | 89 (37.4) | |
| NYHA IV | 580 (57.3) | 436 (56.3) | 144 (60.5) | |
| Previous HF admissions* | 1.4 (1.2)1 [0-2] | 1.3 (1.2)1 [0-2] | 1.5 (1.3)1 [1-2] | .023.023 |
| Baseline treatment | ||||
| Beta-blockers | 749 (79.3) | 552 (77.4) | 197 (84.9) | .014 |
| ACEI/ARB | 437 (46.2) | 322 (45.2) | 115 (49.4) | .272 |
| ARNI | 222 (23.8) | 158 (22.5) | 64 (27.8) | .103 |
| MRA | 797 (84.1) | 600 (84.0) | 197 (84.2) | .955 |
| Loop diuretics | 889 (93.7) | 662 (92.6) | 227 (97.0) | .016 |
| Thiazides | 178 (18.8) | 125 (17.6) | 53 (22.7) | .079 |
| Acetazolamide | 7 (0.8) | 5 (0.7) | 2 (0.9) | .685 |
| Digoxin | 154 (16.3) | 120 (16.8) | 34 (14.6) | .422 |
| Amiodarone | 274 (27.1) | 199 (25.8) | 75 (31.5) | .082 |
| Hydralazine/nitrates | 33 (3.5) | 21 (2.9) | 12 (5.2) | .111 |
| Ivabradine | 173 (18.3) | 118 (16.6) | 55 (23.7) | .015 |
| SGLT2i | 46 (4.9) | 30 (4.2) | 16 (6.9) | .098 |
| PD5i | 91 (9.7) | 61 (8.6) | 30 (13.1) | .043 |
| Statins | 485 (51.3) | 358 (50.1) | 127 (54.7) | .223 |
| Anticoagulation | 631 (66.5) | 476 (66.6) | 155 (66.2) | .931 |
| ERA | 24 (2.6) | 17 (2.4) | 7 (3.0) | .592 |
| Devices | ||||
| ICD | 781 (77.1) | 576 (74.2) | 205 (86.5) | <.001 |
| Cardiac resynchronization therapy | 263 (26) | 188 (24.2) | 75 (31.6) | .023 |
| Percutaneous edge-to-edge MVR | 32 (3.4) | 17 (2.4) | 15 (6.4) | .003 |
| Laboratory tests | ||||
| Hemoglobin, g/L | 13.3±2.1 | 13.3±2.1 | 13.2±2.0 | .614 |
| Creatinine, mg/dL | 1.28±0.54 | 1.25±0.50 | 1.39±0.63 | .001 |
| Urea, mg/dL | 69.2±38.1 | 66.8±36.5 | 76.4±41.8 | .011 |
| Na, mmol/L | 138.2±4.2 | 138.2±4.3 | 138.2±3.7 | .799 |
| K, mmol/L | 4.2±0.5 | 4.2±0.5 | 4.2±0.5 | .657 |
| AST, U/L | 29.5±23.3 | 30.4±25.8 | 26.6±12.2 | .005 |
| ALT/GPT, U/L | 30.4±31.6 | 31.1±34.1 | 28.4±21.7 | .307 |
| GGT, U/L | 103.7±108.4 | 103.5±110.8 | 104.6±100.6 | .902 |
| ALP, U/L | 116.5±73.4 | 115.5±74.4 | 119.8±69.9 | .509 |
| Albumin, g/L | 4.1±0.5 | 4.1±0.6 | 4.1±0.5 | .875 |
| Bilirubin, mg/dL | 1.3±0.8 | 1.3±0.8 | 1.3±0.8 | .766 |
| Ferritin, ng/mL | 182.2±149.0 | 175.0±143.7 | 206.3±163.4 | .045 |
| TSI, % | 21.2±12.3 | 21.4±12.5 | 20.5±11.4 | .460 |
| HbA1c, % | 6.0±0.9 | 6.1±0.9 | 6.0±0.8 | .638 |
| NT-proBNP, pg/mL | 5257±16242 | 5373±18408 | 4891±5357 | .728 |
| Hs-TnT, ng/L | 351.2±2898 | 397.2±3180 | 132.4±502.3 | .679 |
| Echocardiography | ||||
| LVEF, % | 27.8±13.1 | 28.7±14.0 | 25.3±9.9 | <.001 |
| LVEDD, mm | 62.8±13.1 | 62.0±13.5 | 65.3±11.1 | <.001 |
| TAPSE, mm | 13.8±5.8 | 14.0±5.9 | 13.4±5.7 | .327 |
| Estimated sPAP, mmHg | 46.7±14.2 | 46.7±14.4 | 46.8±13.6 | .914 |
| Significant MR | 549 (59.0) | 401 (57.3) | 148 (64.1) | .069 |
| Significant TR | 434 (47.6) | 322 (47.1) | 112 (48.9) | .644 |
| Estimated CVP | ||||
| Normal | 293 (39.4) | 234 (41.3) | 59 (33.5) | .020 |
| Mildly elevated | 245 (33.0) | 188 (33.2) | 57 (32.4) | |
| Severely elevated | 205 (27.6) | 145 (25.6) | 60 (34.1) | |
| Right heart catheterization | ||||
| sPAP, mmHg | 44.7±15.8 | 44.3±15.6 | 46.0±16.4 | .173 |
| mPAP, mmHg | 29.2±9.8 | 28.8±9.6 | 30.4±10.2 | .037 |
| dPAP, mmHg | 21.0±7.9 | 20.7±7.7 | 22.0±8.5 | .030 |
| PCWP, mmHg | 20.6±8.1 | 20.4±8.0 | 21.4±8.4 | .085 |
| CO, L/min | 4.1±1.2 | 4.1±1.2 | 4.2±1.5 | .399 |
| TPG, mmHg | 8.6±4.7 | 8.5±4.3 | 9.0±5.6 | .190 |
| PVR, WU | 2.2±1.3 | 2.2±1.3 | 2.3±1.3 | .726 |
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ALP, alkaline phosphatase; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; AST, aspartate aminotransferase; BMI, body mass index; CVP, central venous pressure; CO, cardiac output; dPAP, diastolic pulmonary artery pressure; ERA, endothelin receptor antagonist; HbA1c, glycated hemoglobin; HF, heart failure; Hs-TnT, high sensitivity troponin T; HT, heart transplantation; ICD, implantable cardioverter defibrillator; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; MVR, mitral valve repair; NYHA, New York Heart Association; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PCWP, pulmonary capillary wedge pressure; PD5i, phosphodiesterase type 5 inhibitor; PVR, pulmonary vascular resistance; SGLT2i, sodium-glucose cotransporter 2 inhibitor; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TPG, transpulmonary gradient; TSI, transferrin saturation index; WU, Wood units.
Values are expressed as mean±standard deviation, No. (%), or median [interquartile range].
Compared with the control group, patients requiring levosimendan had more frequent HF admissions during the previous year, a higher proportion had an implantable cardioverter defibrillator (86.5% vs 74.2%; P <.001), cardiac resynchronization therapy (31.6% vs 24.2%; P=.023), percutaneous edge-to-edge mitral valve repair (6.4% vs 2.4%; P=.003) and higher median (30.4 vs 28.8mmHg; P=.037) and diastolic (22 vs 20.7mmHg; P=.030) pulmonary pressures. Patients in the control group were more frequently female (29.3% vs 22.3%; P=.033), had lower requirements for loop diuretics (92.6% vs 97.0%; P=.016), had less beta-blockers (77.4% vs 84.9%; P=.014), lower levels of serum creatinine (1.25±0.5 vs 1.39±0.6mg/dL; P=.001) and higher left ventricular ejection fraction (28.7±14.0 vs 25.3±9.9%; P <.001). No statistically significant differences were found in age, functional class, or NT-proBNP levels.
Patterns of levosimendan infusionTable 2 shows the main features of levosimendan infusion patterns across centers. More than half of the patients started levosimendan within the first 30 days after inclusion (median, 29 days; interquartile range [IQR]: 10-119) and 55.5% had received levosimendan previously in the context of acute HF hospitalization. There was wide heterogeneity in dose patterns, but fixed doses were more frequently prescribed than weight-adjusted doses. The most frequent dose was 6.25mg per session (n=54, 22.7%) followed by 12.5mg per session (n=51, 21.4%) and 0.1μg/kg/min in 24hours (n=50, 21.0%). Most patients received flexible infusions according to clinical requirements during follow-up (n=136, 57.1%) instead of stable regimes (fixed or unlimited). Bi-weekly infusions were used in 49.5% of patients in stable regimes.
Patterns of levosimendan infusion
| Variables | Levosimendan(N=238) |
|---|---|
| Dosing | |
| 2.5 mg/session | 38±16.0 |
| 3.25 mg/session | 1±0.4 |
| 6.25 mg/session | 54±22.7 |
| 12.5 mg/session | 51±21.4 |
| 0.1μg/kg/min-24 h | 50±21.0 |
| 0.2μg/kg/min-6 h | 37±15.5 |
| Unknown | 7±2.9 |
| Cumulative doses | |
| Cycles per patient | 6.4±9.1 |
| Overall dose administered, mg/kg | 0.4±0.5 |
| Initial strategy | |
| Flexible (as needed) | 136 (57.1) |
| Fixed number of doses | 10 (4.2) |
| Unlimited | 85 (35.7) |
| Unknown | 7 (2.9) |
| Frequency (in fixed and unlimited strategies) | |
| Weekly | 2 (2.1) |
| Every 2 wk | 47 (49.5) |
| Every 3 wk | 4 (4.2) |
| Every 4 wk | 7 (7.4) |
| Other | 3 (3.2) |
| Unknown | 32 (33.7) |
| Discontinuation | 48 (20.2) |
| Futility | 27 (11.3) |
| Improvement | 12 (5.0) |
| Adverse events | 5 (2.1) |
| Hypotension | 2 (0.8) |
| Ventricular arrhythmia | 2 (0.8) |
| Supraventricular arrhythmias | 1 (0.4) |
| Unknown | 4 (1.6) |
Values are expressed as mean±standard deviation or No. (%).
Levosimendan infusions were well tolerated with a low proportion of patients (n=5, 2.1%) experiencing adverse events that led to discontinuation. In detail, 2 patients (0.8%) had symptomatic hypotension, 2 patients (0.8%) had nonfatal ventricular arrhythmias, and 1 patient (0.4%) had supraventricular arrhythmias. In addition, 27 patients (11.3%) discontinued levosimendan due to a lack of clinical response, and 12 patients (5%) due to significant clinical improvement. Overall, 20.2% of patients discontinued treatment during follow-up. Discontinuation was less frequent in patients receiving the drug in a flexible-dose pattern compared with other patterns [9 (7.1%) vs 40 (40.2%)], mostly due to futility (table 4 of the supplementary data).
Follow-upOverall, the patients were on the waiting HT list for a median of 4.2 [1.4-9.1] months. A total of 935 patients (92.1%) received HT during follow-up, 8 patients (0.8%) were on the waiting list at the last evaluation, 33 patients (3.3%) died while on the waiting list, and 39 patients (3.8%) were excluded due to new-onset contraindications (n=22) or due to significant clinical improvement (n=17). No patients were lost to follow-up. Patients who received levosimendan had a significantly longer waiting time for HT (169 [IQR 72-292] days vs 103 [IQR 39-241] days, P <.001).
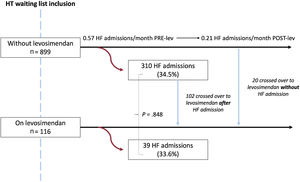
Heart failure admissions, mechanical circulatory support, and survival on the waiting listFigure 2 displays a flowchart of HF admissions according to the time of levosimendan initiation. The proportion of patients requiring hospitalization did not significantly differ between patients who started levosimendan in the first 30 days after inclusion and those who did not (n=39; 33.6% vs n=310; 34.5%; P=.848). In the latter group, almost one-third (n=102; 32.9%) crossed over to the levosimendan group after an HF admission, and the HF admission rate decreased from 0.57 to 0.21 HF admissions per month after levosimendan initiation. Total rates of HF admissions while patients were on the waiting list were similar among those who never received levosimendan, those who initiated the treatment in the first 30 days after inclusion, and those who started the treatment later (0.21, 0.24, and 0.26, respectively).
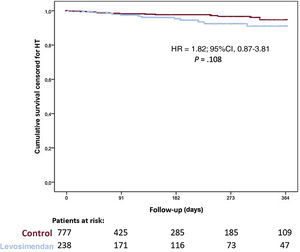
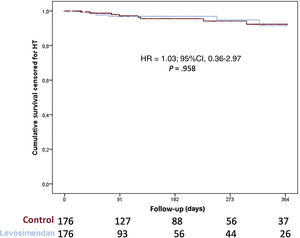
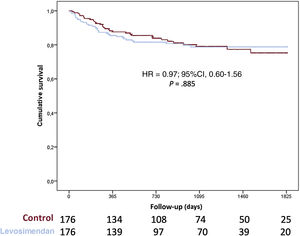
Overall, 169 patients (16.6%) required mechanical circulatory support while on the waiting list and 133 patients (13.1%) required urgent mechanical circulatory support due to acute cardiogenic shock (35 left Centrimag, 13 biventricular Centrimag, 25 veno-arterial ECMO, 30 intra-aortic balloon pump, and 32 Impella), 36 patients (3.5%) required long-term left ventricular assist devices (LVAD) due to chronic worsening HF. Urgent HT was required in 170 patients and no differences were found between groups in the proportion of patients requiring urgent HT (20.0% vs 17.7%; P=.445). Figure 3 shows 1-year survival on the waiting list among patients who received levosimendan and the control group. No significant differences were observed between groups (hazard ratio [HR], 1.82; 95%CI, 0.87-3.81; P=.108). When 1-year survival on the waiting list was analyzed by propensity score matching, there were no differences between groups (HR, 1.03; 95%CI, 0.36-2.97; P=.958) as shown in figure 4. No significant differences were observed in 1-year survival on the waiting list among prescription patterns (figure 1 of the supplementary data).
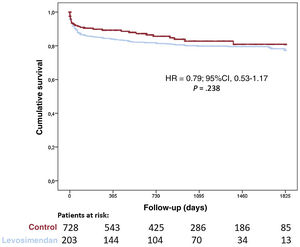
A significant proportion of patients (n=203; 21.7%) had primary graft failure after HT and 167 patients (17.9%) required mechanical circulatory support. No differences between groups were found in primary graft failure (17.1% vs 23.0%; P=.068) or mechanical circulatory support implantation (18.0% vs 17.8%; P=.937). Survival after HT was similar among groups (HR, 0.79; 95%CI, 0.53-1.17; P=.238; and see figure 5) even after adjustment by propensity score matching (HR, 0,97; 95%CI, 0.60-1.56; P=.958; and see figure 6).
The main findings of our study (figure 7), which represents most of the patients included on the elective HT waiting list at most centers in Spain, with more than 1000 patients, and 5 years of follow-up, were as follows: a) almost one-quarter of HT recipients received ambulatory infusions of levosimendan while on the waiting list; b) these patients tended to have a worse clinical profile (more frequent HF admissions during the previous year, lower left ventricular ejection fraction, higher pulmonary pressures, and worse renal function); c) the prescription patterns varied widely across centers, with the most frequent pattern being fixed doses according to clinical requirements; d) levosimendan seemed to be a safe treatment in this population with <1% of patients experiencing severe adverse events; e) no differences in survival were found between patients on levosimendan vs controls before or after HT, after adjustment by propensity score matching; f) HF hospitalizations may be reduced.
HT is still the best alternative for patients with AHF when conventional treatment and devices fail.5,19,20. However, while patients are on the waiting list, the risk of HF readmission and mortality is high and apart from an LVAD, there are limited options for their management, including cardiac resynchronization therapy and percutaneous edge-to-edge mitral valve repair, and evidence comes mostly from observational studies.21,22 Therefore, given that not all patients are candidates for an LVAD or percutaneous edge-to-edge mitral valve repair, there is a need for alternative treatments in this scenario. Inotropic drugs have been used in patients with AHF to improve symptoms but there is concern about their safety.23 Ambulatory infusions of levosimendan have emerged as an option in some centers due to their potential advantages compared with classic catecholaminergic drugs. This treatment confers an increase in myocardial contractility without increasing intracellular calcium, can be used in patients on beta-blockers, and decreases pulmonary pressures; a particular feature is that its action, mediated by the active metabolite OR-1896, has a prolonged effect extending beyond the time of administration for several days, thus avoiding the need for cumbersome continuous intravenous infusions..24,25. This particular levosimendan treatment modality has shown potential clinical and hemodynamic benefits in patients with AHF, as well as improvement in neurohormonal markers.14–17 While ambulatory levosimendan infusions in day hospitals are an off-label treatment scheme, this modality has been adopted in many HF programs in some European countries, including Spain, as a palliative measure to reduce HF hospitalizations and improve quality of life.12–15 However, data on its use in patients on the waiting list as a bridge to HT are scarce.26–28
In our registry, we observed that almost a quarter of the patients received this treatment while on the waiting list. The factors prompting clinicians to start this treatment as a bridge to HT seemed to be an initially worse clinical picture or an HF hospitalization while the patient was on the waiting list. Due to a lack of consensus on the best levosimendan infusion pattern in this setting, centers vary widely in how they administer this treatment. Although the evidence is greatest for a fixed number of sessions with weight-adjusted doses, prescription according to patient requirements and in a fixed dose are the most widely used modalities in clinical practice, due to its simplicity.14,17 An important finding of our registry is that despite the AHF situation of patients receiving levosimendan (INTERMACS 3), the safety of levosimendan was notable, with only 0.8% of patients having a nonfatal ventricular arrhythmia while they were receiving intermittent levosimendan infusions, as most of the patients had an implantable cardioverter defibrillator that could be reviewed. Another advantage of using levosimendan is that its inotropic effect is unaffected by the use of beta-blockers, which may have implications in the prognosis of patients with HF. 25,29 In this study, more patients were on beta-blockers in the levosimendan arm (84.9% vs 77.4%; P=.014).
Although patients receiving levosimendan spent more than 2 months on the waiting list, this was not reflected in terms of clinical events. Mortality on the waiting list was low in our series, in accordance with previously described reports of the Spanish Heart Transplant Registry. 630 Although there was a trend to higher mortality in patients with levosimendan on the waiting list, which can also be explained by their worse clinical profile, we found no differences between groups at 1 year even when we performed an analysis by propensity score matching. Regarding HF admissions, we performed 2 analyses. First, we compared patients who initiated levosimendan at the beginning of the study (in the first month on the waiting list, as they represented more than half of patients with this treatment) with patients who did not, and found no differences in HF admissions. However, an interesting observation was that, in the latter group, 102 patients crossed over to levosimendan after a HF admission, and only 20 started this treatment without an HF admission. Therefore, initiating levosimendan could be a marker of risk as it usually seems to be triggered by an HF admission. Second, in the group that did not receive levosimendan during the first month on the waiting list, we analyzed the HF admissions rate of those who later received ambulatory infusions. The HF admissions rate was higher (0.57 per month) prior to levosimendan initiation compared with after levosimendan initiation (0.21 per month). There were no differences in the need for MSC or urgent HT. Finally, 92% of the patients included in our study received an HT, and receiving levosimendan while on the waiting list did not influence their survival before or after HT.
LimitationsFirst, this study has the limitations inherent to its observational design and retrospective collection of data on the use of levosimendan. However, all the data on the pre-HT situation and post-HT outcomes had been previously collected in the national transplantation registry and were transferred directly to our database. Because patients who received levosimendan were not as sick as patients not receiving the drug, we performed propensity score matching to compare survival between the groups. Given the retrospective nature of our data, our aim was not to compare the 2 groups, but rather to evaluate the safety of this treatment.
Second, when patients are treated with ambulatory levosimendan infusions, other intravenous treatments such as furosemide or iron may also have been prescribed and this information was not available. The follow-up during infusions tends to be closer, which may help detect HF decompensations and better adjust diuretics and HF medication.
Third, among patients who received levosimendan, 55% had a previous infusion during a prior hospitalization, which could potentially have led to selection of patients without previous adverse events.
Finally, limitations inherent to our registry data are the lack of a standardized algorithm on when and how to initiate levosimendan, the heterogeneity of prescription patterns (with no administration study protocol), and the varying lengths of levosimendan exposures among patients.
CONCLUSIONSRepetitive ambulatory infusions of levosimendan in patients on the elective HT waiting list is a common clinical practice, with heterogeneity in prescription patterns. Patients receiving levosimendan tended to have a worse clinical profile and remained longer on the waiting list. Severe adverse events were rare, mortality was not increased, and HF hospitalizations may be reduced in patients receiving levosimendan compared with those that did not.
- –
Levosimendan is an inotropic drug that can improve signs and symptoms of HF.
- –
Ambulatory prescription of levosimendan in patients on AHF is common in some centers, but there is some concern about patient profiles, safety, and efficacy.
- –
In patients on the HT waiting list, levosimendan seems to be safe, even though these patients tend to have a worse clinical profile.
- –
Levosimendan may be considered as a treatment option in patients with AHF to promote clinical stability while they are on the HT waiting list.
This was an investigator-initiated study and received financial support from Orion Pharma for creating the online data base and for performing the study analysis. The design and analysis of the data, as well as drafting the manuscript was performed independently by the investigators.
AUTHORS’ CONTRIBUTIONSJ. de Juan Bagudá and F. de Frutos contributed equally as first authors. All authors contributed to the collection of data, analysis, manuscript drafting, and its critical review.
CONFLICTS OF INTERESTJ. de Juan Bagudá has received honoraria for lectures from Orion Pharma. J. Guzman-Bofarull has received support for attending meetings from Sanofi, Bayer, Bristol, Pfizer, Novartis, Rovi, and Boehringer-Ingelheim. C. Mitroi has received honoraria for lectures from Pfizer, Lilly, Daiichi-Sankyo and Abbott; support for attending meetings from Pfizer, Rovi, Lilly, and Bayer. M.D. García-Cosío Carmena has received honoraria for lectures from AstraZeneca and Chiesi; support for attending meetings from Abbot and Rovi. D. Dobarro has received honoraria for lectures from Orion Pharma, Boehringer-Ingelheim, AstraZeneca and Novartis; participation in advisory boards from Orion Pharma, Astra Zeneca, Boehringer-Ingelheim, and Novartis. F. González-Vílchez has received honoraria for lectures from Novartis; support for attending meetings from Pfizer. J. González-Costello has received honoraria for lectures from Abbott, Orion Pharma, Pfizer, Alnylam, Boehringer-Ingelheim, Zoll, Rovi and AstraZeneca; participation in advisory boards from Abbott, Chiesi, Pfizer, Alnylam, Novartis, Bayer, and AstraZeneca; support for attending meetings from Abbott, AstraZeneca, Zoll, and Alnylam. The remaining authors declare that they have no conflicts of interest relevant to the content of this manuscript.