Risk stratification of ventricular arrhythmias in patients with repaired tetralogy of Fallot (rTOF) remains unresolved. We aimed to identify right ventricular (RV) electrophysiological parameters potentially associated with a higher risk of ventricular arrhythmias in patients with rTOF.
MethodsWe included all consecutive patients with rTOF who underwent RV electroanatomical mapping at a single tertiary center. We used logistic regression modeling to identify those variables associated with an increased risk of clinical or induced ventricular tachycardia (VT), or clinical VT exclusively.
ResultsTwenty-one of the 56 patients included had clinical or induced VT. A high-frequency of premature ventricular contractions/nonsustained VT (OR, 11.34; 95%CI, 1.50-85.97; P=.019), an HV interval > 55 ms (OR, 21.20; 95%CI, 3.12-144.14; P=.002), and RV activation time (ms) (OR [per 10ms intervals], 1.34; 95%CI, 1.02-1.75; P=.035) proved to be associated with clinical or induced VT. The model including this information had good discrimination ability, with an area under the curve of 0.884 (95%CI, 0.79-0.97; P <.001). When considering only clinical VT as the outcome of interest, only an HV interval > 55ms (OR, 9.65; 95%CI, 1.41-66.14; P=.021) and high-frequency of premature ventricular contractions/nonsustained VT (OR, 13.14; 95%CI, 1.95-88.54; P=.008) were independently associated (area under the curve of 0.836 [95%CI, 0.663-1.000; P=.002]).
ConclusionsHigh-frequency of premature ventricular contractions/nonsustained VT, an HV interval> 55ms and RV activation time are factors associated with an increased risk of ventricular arrhythmias in patients with rTOF.
Keywords
Repaired tetralogy of Fallot (rTOF) constitutes the largest group of adults with repaired cyanotic congenital heart disease, with an estimated prevalence of around 0.23‰ of inhabitants.1,2 Despite good long-term survival, residual lesions lead to an increase in morbidity and mortality in adulthood compared with the general population.
Sudden cardiac death is one of the leading causes of death in this population, with an estimated incidence of 1.4 per 1000 person-years.3,4 Moreover, a high prevalence (14.6%) of nonfatal ventricular arrhythmias (VA), mostly monomorphic ventricular tachycardia (VT), has been documented in adults with rTOF.5
Although several risk factors have been described in this population, there is still controversy regarding the best predictors of VA and sudden cardiac death.6–8 Recent studies suggest that right ventricle (RV) electroanatomical mapping (EAM) could be useful to identify substrates of higher arrhythmogenic risk.9,10 However, evidence in the setting of rTOF remains scant.
The aim of this study was to identify clinical and electrophysiological parameters of the RV associated with a higher risk of VA in a selected high-risk population of patients with rTOF.
METHODSStudy populationThis is an ambispective study including all consecutive patients with rTOF who were referred for an electrophysiological study (EPS) and an RV EAM at a single tertiary referral center from July 2006 to July 2017. Most patients (n=50) were recruited prospectively at the time of EPS (November 2012 to July 2017); additionally, 6 patients were recruited retrospectively (March 2006 to October 2012). Following our standard clinical protocol, indications for EPS and RV EAM in rTOF patients were documented spontaneous VT, clinical suspicion of VT, and risk stratification (with potential ablation) prior to pulmonary valve replacement. The study followed the national and international guidelines (2013 Declaration of Helsinki) and was approved by the Ethics Committee of the Vall d’Hebron Research Institut (VHIR). All patients provided specific informed consent.
Clinical, echocardiographic, cardiac magnetic resonance, baseline (nonpaced) electrocardiogram, EPS and EAM data were collected. Information on nonsustained ventricular tachycardia (NSVT) or high-frequency of premature ventricular contractions (Hi-PVC) (> 30 per hour)11 was retrieved from electrocardiogram recordings, 24-hour Holter monitoring or implantable cardiac defibrillator interrogation.
Electrophysiological study and right ventricle electroanatomical mappingAntiarrhythmic drugs were stopped 5 half-lives before the procedure. EPS were performed under superficial conscious sedation and through femoral vein access. Atrioventricular conduction was evaluated at baseline. Programmed electrical stimulation for VT inducibility was performed at 3 paced cycle lengths (600/500/400ms) followed by up to 3 extra stimuli down to the refractory period or 200ms, delivered at the RV apex and the infundibular septum, before and during isoproterenol infusion (2-10μg/min). Programmed electrical stimulation was considered positive if sustained VT (monomorphic or polymorphic) or ventricular fibrillation were induced.
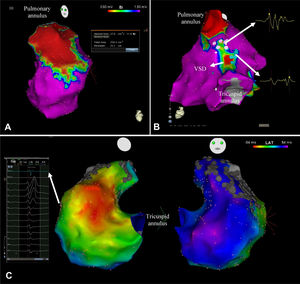
RV 3D-EAM was performed during sinus rhythm, using the QRS as reference, with Ensite Velocity (n=11), Ensite Precision (n=4) (Abbott), Carto XP (n=4) or Carto 3 (n=37) (Biosense Webster). Mapping catheters used were NaviStar (n=41) (Biosense Webster), Cool-Flex (n=14) and Tacticath Quartz (n=1) (Abbott). Electrogram characteristics (peak-to-peak bipolar amplitude and local activation time, defined as sharp peak deflection of the local bipolar electrograms that coincides with the maximum downstroke of the local unipolar signal) at each contact point were recorded and displayed color-coded, for voltage and activation sequence over the RV shell (figure 1). Points were acquired in stable sites and adequate contact with a 15-mm threshold setting. According to previous literature,12 electrograms with a peak-to-peak voltage <1.5mV were defined as low voltage, and areas with at least 3 adjacent points with peak-to-peak voltage <0.5mV were considered scar. Scar areas in the whole RV and RV outflow tract (RVOT) were measured and the percentage of scar was calculated with respect to the total area of the RV. Anatomical isthmi with a voltage amplitude > 0.5 mV between scar areas and the valvular annulus were quantified (figure 1). Fragmented electrograms (FEGM), defined as those with ≥ 4 deflections,> 40ms duration and <1mV voltage,13 and double potentials (DP), defined as those with 2 components separated by an isoelectric line, were labeled (figure 1). To achieve uniform localization of FEGM and DP, the RV was segmented into 7 anatomical areas (RV septum, RV anterior wall, RV posterior wall, ventricular septum defect, septal RVOT, anterior RVOT, and posterior RVOT), and the number of areas with FEGM and DP was calculated. Total RV activation time, defined as the time elapsed between the earliest and the latest electrograms, and infundibular activation time, defined as the time between the septal and lateral edge of the ventricular septal defect were determined.
A: right ventricle outflow tract scar area (in red) on the voltage map of a patient repaired with a transannular patch. B: posterior view of a right ventricle voltage map showing an area of scar at the VSD. Double potentials and fragmented electrograms are labeled in the map with blue and pink dots respectively. C: activation map of a patient with a right ventricle activation time of 108ms. VSD, ventricular septal defect.
Variables are reported as mean±SD, median [p25-p75] or frequency (%) where appropriate. Differences between groups were compared using the Fisher exact test, Student t-test or Mann-Whitney U-test where appropriate. The primary endpoint was the presence of spontaneous or induced VA at any time. For better clinical applicability, a secondary endpoint evaluating exclusively the presence of spontaneous VA was also defined. Variables associated with both endpoints were explored using logistic regression analyses. Continuous variables were categorized or stratified for simplification. RV activation time strata were set every 10ms from the entire number closest to the minimum value (60ms). Those showing an association with a P <.1 or thought to be clinically relevant were included in the multivariable model. Several models were tested to select the most parsimonious model containing the smallest number of variables that maximized the discrimination ability assessed by the area under the receiver operating characteristic curve (AUC). Significance for all analyses was set at the level of .05. Statistical analyses were performed with IBM SPSS 20.0 software and RStudio.
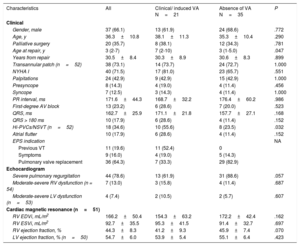
RESULTSStudy populationA total of 56 patients with rTOF were included. Baseline characteristics of the study population are summarized in table 1. A ventricular approach was used in all reparative surgeries. Thirty-eight patients underwent implantation of a transannular patch to expand the RVOT, 3 a RV-to-pulmonary artery conduit, and 15 a ventriculotomy alone. At the time of EPS, 8 patients had already undergone pulmonary valve replacement for severe pulmonary regurgitation and 5 patients a previous ineffective focal ablation. Thirty-nine patients (69%) were symptomatic for palpitations (n=24), presyncope (n=8) or syncope (n=7). Overall, the functional status assessed by the treating clinician was good (NYHA class I in 40 patients, II in 14, and III-IV in 2). Imaging studies confirmed that almost 80% of patients had severe pulmonary regurgitation, although, in the vast majority, left ventricular and RV systolic function were preserved or only mildly depressed.
Clinical characteristics
| Characteristics | All | Clinical/ induced VA N=21 | Absence of VA N=35 | P |
|---|---|---|---|---|
| Clinical | ||||
| Gender, male | 37 (66.1) | 13 (61.9) | 24 (68.6) | .772 |
| Age, y | 36.3±10.8 | 38.1±11.3 | 35.3±10.4 | .290 |
| Palliative surgery | 20 (35.7) | 8 (38.1) | 12 (34.3) | .781 |
| Age at repair, y | 3 (2-7) | 7 (2-10) | 3 (1-5.0) | .047 |
| Years from repair | 30.5±8.4 | 30.3±8.9 | 30.6±8.3 | .899 |
| Transannular patch (n=52) | 38 (73.1) | 14 (73.7) | 24 (72.7) | 1.000 |
| NYHA I | 40 (71.5) | 17 (81.0) | 23 (65.7) | .551 |
| Palpitations | 24 (42.9) | 9 (42.9) | 15 (42.9) | 1.000 |
| Presyncope | 8 (14.3) | 4 (19.0) | 4 (11.4) | .456 |
| Syncope | 7 (12.5) | 3 (14.3) | 4 (11.4) | 1.000 |
| PR interval, ms | 171.6±44.3 | 168.7±32.2 | 176.4±60.2 | .986 |
| First-degree AV block | 13 (23.2) | 6 (28.6) | 7 (20.0) | .523 |
| QRS, ms | 162.7±25.9 | 171.1±21.8 | 157.7±27.1 | .168 |
| QRS > 180 ms | 10 (17.9) | 6 (28.6) | 4 (11.4) | .152 |
| Hi-PVCs/NSVT (n=52) | 18 (34.6) | 10 (55.6) | 8 (23.5) | .032 |
| Atrial flutter | 10 (17.9) | 6 (28.6) | 4 (11.4) | .152 |
| EPS indication | NA | |||
| Previous VT | 11 (19.6) | 11 (52.4) | 0 | |
| Symptoms | 9 (16.0) | 4 (19.0) | 5 (14.3) | |
| Pulmonary valve replacement | 36 (64.3) | 7 (33.3) | 29 (82.9) | |
| Echocardiogram | ||||
| Severe pulmonary regurgitation | 44 (78.6) | 13 (61.9) | 31 (88.6) | .057 |
| Moderate-severe RV dysfunction (n = 54) | 7 (13.0) | 3 (15.8) | 4 (11.4) | .687 |
| Moderate-severe LV dysfunction (n=53) | 4 (7.4) | 2 (10.5) | 2 (5.7) | .607 |
| Cardiac magnetic resonance (n=51) | ||||
| RV EDVi, mL/m2 | 166.2±50.4 | 154.3±63.2 | 172.2±42.4 | .162 |
| RV ESVi, mL/m2 | 92.7±35.5 | 95.3±41.5 | 91.4±32.7 | .697 |
| RV ejection fraction, % | 44.3±8.3 | 41.2±9.3 | 45.9±7.4 | .070 |
| LV ejection fraction, % (n=50) | 54.7±6.0 | 53.9±5.4 | 55.1±6.4 | .423 |
EDVi, end-diastolic volume index; EPS, electrophysiological study, ESVi end-systolic volume index; Hi-PVC, high-frequency premature ventricular contractions; LV, left ventricle; NA, not applicable; NSVT, nonsustained ventricular tachycardia; RV, right ventricle; VA, ventricular arrhythmia.; VT, ventricular tachycardia.
The data are presented as No. (%) or mean±standard deviation.
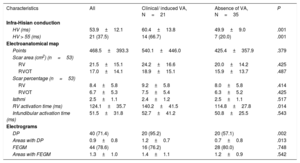
Details of EPS and EAM parameters obtained within the same procedure are shown in table 2. At the time of the EAM, a total of 17 sustained monomorphic VT were induced in 14 patients with a mean cycle length of 291.9±68.5ms (from 200.0 to 410.0ms). No episodes of polymorphic VT or ventricular fibrillation were induced. A pathological infra-Hisian conduction was documented in 21 patients (38%).
Electrophysiological characteristics
| Characteristics | All | Clinical/ induced VA, N=21 | Absence of VA, N=35 | P |
|---|---|---|---|---|
| Infra-Hisian conduction | ||||
| HV (ms) | 53.9±12.1 | 60.4±13.8 | 49.9±9.0 | .001 |
| HV > 55 (ms) | 21 (37.5) | 14 (66.7) | 7 (20.0) | .001 |
| Electroanatomical map | ||||
| Points | 468.5±393.3 | 540.1±446.0 | 425.4±357.9 | .379 |
| Scar area (cm2) (n=53) | ||||
| RV | 21.5±15.1 | 24.2±16.6 | 20.0±14.2 | .425 |
| RVOT | 17.0±14.1 | 18.9±15.1 | 15.9±13.7 | .487 |
| Scar percentage (n=53) | ||||
| RV | 8.4±5.8 | 9.2±5.8 | 8.0±5.8 | .414 |
| RVOT | 6.7±5.3 | 7.5±5.4 | 6.3±5.2 | .425 |
| Isthmi | 2.5±1.1 | 2.4±1.2 | 2.5±1.1 | .517 |
| RV activation time (ms) | 124.1±35.7 | 140.2±41.5 | 114.8±27.8 | .014 |
| Infundibular activation time (ms) | 51.5±31.8 | 52.7±41.2 | 50.8±25.5 | .543 |
| Electrograms | ||||
| DP | 40 (71.4) | 20 (95.2) | 20 (57.1) | .002 |
| Areas with DP | 0.9±0.8 | 1.2±0.7 | 0.7±0.8 | .013 |
| FEGM | 44 (78.6) | 16 (76.2) | 28 (80.0) | .748 |
| Areas with FEGM | 1.3±1.0 | 1.4±1.1 | 1.2±0.9 | .542 |
FEGM, fragmented electrograms; DP, double potentials; RV, right ventricle; RVOT, right ventricle outflow tract; VA, ventricular arrhythmia; VSD, ventricular septal defect.
The data are presented as No. (%) or mean±standard deviation.
High-density EAM (mean 469 points) revealed that all patients had a RV scar area embracing a mean of 8% of the RV tissue (scar extension could not be calculated in 3 cases due to technical reasons). In most patients, scar areas were located in the regions affected by the reparative surgery (RVOT, ventricular septal defect, and RV anterior wall). Other locations of scar (inferior wall and other septum locations) were infrequent (11%). All patients showed at least 1 anatomical isthmus, with a mean of 2.5 isthmi per patient.
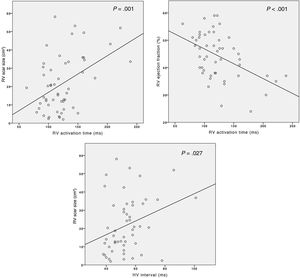
Mean RV activation time and infundibular activation time were 124.1±35.7ms and 51.5±31.8ms, respectively. RV activation time was correlated with both anatomical and functional parameters of the RV (figure 2), exhibiting a positive correlation with scar size (r=0.455, P=.001) and a negative correlation with RV ejection fraction (r=-0.475, P <.001). Likewise, scar size was similarly correlated with the HV interval (r=0.307, P=.027, figure 2).
The prevalence of DP and FEGM for the overall population was high (71% and 79%, respectively) and only 3 patients did not have pathological electrograms. For the overall population, DP and FEGM were found in a mean of 0.9±0.8 and 1.3±1.0 anatomical areas, most frequently at RVOT and surrounding ventricular septal defect.
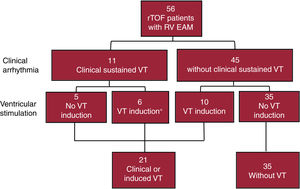
Variables associated with VATwenty-one out of the 56 patients met the primary endpoint of spontaneous or induced VA: 11 had clinical VT (one of whom also had an episode of recovered sudden cardiac death), and in 10 additional patients, with no prior VA, a sustained VT was inducible in the EPS (figure 3). Four of the 11 patients with clinical VT were also inducible at the time of the EAM, and 2 additional patients had been inducible in a previous EPS. An implantable cardiac defibrillator had been implanted prior to EPS in 5 of the patients. The remaining 35 patients comprised the group without VA.
Univariate analysis (table 1) showed that patients with VA were older at the time of surgical repair (median age: 7 vs 3 years, P=.047) and had a higher prevalence of Hi-PVC/NSVT (56% vs 24%, P=.032). RV systolic function measured by cardiac magnetic resonance tended to be lower in the group with VA, although RV dimensions were similar between groups.
No significant differences were observed between the 2 groups regarding age at the time of evaluation, time since intervention, left ventricular function, history of palliative surgery, use of transannular patch, arrhythmia-related symptoms, and baseline electrocardiogram parameters.
Infra-Hisian conduction time was significantly longer in the group of patients with VA (HV interval 60.4±13.8 vs 49.9±9.0ms, P=.001), with a higher proportion of patients with infra-Hisian conduction disturbance (67% vs 21%, P=.001, table 2). RV and RVOT scar extensions were similar in the 2 groups. RV activation time was significantly longer in the group with VA (140.2±41.5 vs 114.8±22.8ms, P=.014), although no significant differences between groups were observed in infundibular activation time (52.74±41.23 vs 50.76±35.51, P=.543). Patients with VA had a significantly higher prevalence of DP (95% vs 57%, P=.002) and a higher number of anatomical areas with DP (1.24±0.7 vs 0.71±0.75, P=.013). However, no differences were observed in the prevalence of FEGM and the mean number of areas with FEGM (table 2).
From those variables showing an association with the primary endpoint in the univariable analysis, the most parsimonious multivariate model was built using the minimum number of variables with the highest discrimination ability. The final model included the following variables (table 3): an HV interval> 55ms (OR, 21.20; 95%CI, 3.11-144.14; P=.002); the presence of Hi-PVC/NSVT (OR, 11.33; 95%CI, 1.50-85.97; P=.019); and RV activation time (OR [per 10ms interval], 1.34; 95%CI, 1.02-1.75; P=.035). A model including this information had a discrimination ability, assessed by the AUC, of 0.884 (95%CI, 0.79-0.97; P <.001). Other factors such as age at surgical repair, the presence of DP and the number of areas with DP, associated with VA in the univariate analysis, were not confirmed to be independently associated in the multivariate analysis.
Multivariable model
| Clinical or induced VA | |||
| Variable | OR | 95%CI | P |
| Hi-PVCs/NSVT | 11.33 | 1.50-85.97 | .019 |
| HV > 55 ms | 21.20 | 3.11-144.14 | .002 |
| Stratified RV activation time(10ms intervals) | 1.34 | 1.02-1.75 | .035 |
| Clinical VA | |||
| Variable | OR | 95%CI | P |
| Hi-PVCs/NSVT | 13.14 | 1.95-88.54 | .008 |
| HV > 55 ms | 9.65 | 1.41-66.14 | .021 |
95%CI, 95% confidence interval; Hi-PVC, high-frequency premature ventricular contractions; NSVT, nonsustained ventricular tachycardia; OR, odds ratio; RV, right ventricle; VA, ventricular arrhythmia.
As a secondary endpoint, and to provide more clinically relevant guidance, we also evaluated the variables potentially associated exclusively with clinical VA (n=11). Of note, VT inducibility in this case was considered as one of the predictors of interest. The results of the univariate analysis are shown in the . In the multivariate model (table 3), only Hi-PVC/NSVT (OR, 13.14; 95%CI, 1.95-88.54; P=.008), and an HV interval> 55ms (OR, 9.65; 95%CI, 1.41-66.14; P=.021) proved to be independently associated with spontaneous VT. The model including these variables had a discrimination power, evaluated by the AUC, of 0.836 (95%CI, 0.663-1.000; P=.002).
DISCUSSIONVA are a common complication in adults with rTOF that increase morbidity and may even lead to sudden cardiac death.3–5,14 In addition, patients with rTOF and an implantable cardiac defibrillator have a high rate of inappropriate therapies.7 Therefore, risk assessment of VA is crucial to apply effective preventive measures. The present study shows that EPS and RV EAM provide valuable information for the evaluation of arrhythmic risk in this population. Specifically, an HV> 55ms, Hi-PVC/NSVT and RV activation time at the EAM were associated with spontaneous or induced VA. Moreover, when only clinical events were considered as the outcome of interest, an HV> 55ms and Hi-PVC/NSVT were also independently associated.
Invasive studies have shown that most VTs in rTOF patients are secondary to a macro-reentrant mechanism involving the RV.15 Surgical scars, anatomical barriers and myocardial tissue injury constitute the perfect arrhythmogenic substrate. As expected, considering the type of surgical approach during reparative surgery, all patients in our series had an RV scar and ≥ 1 anatomical isthmi. Most of these scars were located in regions affected by previous surgery and other areas of the RV were involved in only a few cases (11%). Although it would seem reasonable to find a relationship between scar extension and arrhythmogenic propensity, no significant differences were observed between the group of patients with clinical or induced VA and the group without arrhythmias. This differs from previous findings using imaging techniques for the evaluation of dense fibrosis. Babu-Narayan et al.16 found an association between ventricular fibrosis extension, evaluated by cardiac magnetic resonance, and arrhythmic risk in rTOF patients. However, in this case, the authors considered atrial arrhythmias as an arrhythmic event and, on the other hand, an exact correlation between the scar evaluated by cardiac magnetic resonance imaging and EAM cannot be assumed in our context. In this regard, similar to the present analysis, Drago et al.10 reported no association between low-voltage area and VT inducibility in a sample of rTOF patients who underwent RV EAM.
Interestingly, other parameters of the RV EAM were associated with the risk of VA in our cohort. A longer RV activation time was associated with clinical or induced VA in the multivariate analysis (OR [per 10ms interval], 1.34; 95%CI, 1.02-1.75; P=.035). Slowed ventricular conduction is a manifestation of a damaged myocardium, which is congruent with the finding of a positive correlation between this variable and the extent of the scar. From a pathophysiological point of view, a slower activation of the RV in sinus rhythm could be the consequence of the existence of areas of slow electrical conduction that can be the substrate of reentrant VT. This is consistent with the results published by Kapel et al.9 showing that RV arrhythmogenic isthmi in patients with rTOF had lower conduction velocities than those that were nonarrhythmogenic. It should be noted that isthmus conduction velocity can be difficult to measure due to the complexity of delimiting the isthmus edge. This is not the case with total RV activation time, which can be easily obtained from the RV sinus map. Infundibular activation time, where the most frequently arrhythmogenic isthmus is located, was not significantly longer in patients with VA. This may be related to the fact that RV activation time, unlike infundibular activation time, reflects the involvement of the overall RV myocardium. Indeed, a negative correlation between RV function and activation time was proven, showing that those patients with a globally sicker RV have certain electrophysiological characteristics that make them more vulnerable to VA.
FEGM and DP could be considered as the electroanatomical expression of the presence of slow-conducting and functional or anatomical block areas. However, neither the presence of FEGM nor its anatomical extension were associated with the risk of VA. FEGMs are a focal expression of tissue damage. However, VA in patients with rTOF, unlike ischemic heart disease, is not due to small channels embedded in the scar, but rather to a macrore-entrant phenomenon that involves larger anatomical isthmi in which the low velocity of conduction is not manifested by this type of local potential. Although in the univariate analysis the prevalence of DP and the mean number of areas with DP were significantly higher in the group with clinical or induced VT, they were not independently associated with VA in the final model. In fact, DP may also represent electrical conduction block that can even prevent the appearance of re-entrant arrhythmias.
One of the major findings of this study is that patients with VA had a significantly longer HV interval. Indeed, an abnormal HV interval was associated with clinical or induced VA (OR, 21.20; 95%CI, 3.12-144.14; P=.002) and also with clinical VA (OR, 9.65; 95%CI, 1.41-66.14; P=.021). Information in the literature about the possible association between abnormal AV conduction and VA in rTOF is scant. Garson et al.17 studied a group of 27 patients with rTOF undergoing EPS and observed that those patients with inducible either sustained VT or NSVT had a significantly longer HV interval. In addition, Kimura et al.18 observed that first-degree AV block and PR interval prolongation (≥ 2ms/y) were risk factors independently associated with lethal VA in a cohort of 176 patients with rTOF. However, in our series neither the PR interval nor the first-degree AV block were associated with VA. In addition to the effect of the sample size, the HV interval could be a more sensitive parameter since, unlike the PR interval, it is not affected by the variability of nodal conduction. Reasons for the association between prolonged infra-Hisian conduction and VT can be diverse. A prolonged HV interval could be the consequence of more aggressive reparative surgery and therefore may reflect a more extensive lesion of the ventricular myocardium. This is consistent with the finding of a significant positive correlation between the extent of scar tissue and the HV interval (r=0.307; P=.027). Furthermore, impaired infra-Hisian conduction could lead to ventricular asynchrony and systolic dysfunction, which increases the risk of VA. In this regard, PR interval and RV enlargement and dysfunction were significantly correlated in the study by Kimura et al.18 In the present study, RV systolic function tended to be lower in the group with clinical or induced VT, although not reaching statistical significance.
Concerning clinical variables, we could only find an association between Hi- PVC/NSVT and VT. From a pathophysiological point of view, it is reasonable to speculate that Hi-PVC/NSVT may be the trigger for sustained VT. Moreover, this finding is consistent with previous published data.5,6,19 Although the small sample size prevents us from definitive conclusions in this regard, the strength of the association between Hi-PVC/NSVT and the outcome of interest indicates that it is perhaps the most relevant clinical information for predictive purposes. The lack of association between RV dysfunction and VT is notable, although RV systolic function tended to be lower in the group with VA and was related to the 2 identified electrophysiological risk factors (RV activation time and abnormal infra-Hisian conduction). This highlights the greater predictive accuracy of electrophysiological markers for risk stratification of VA in the rTOF population.
To the best of our knowledge, this is the first study that establishes 2 invasive parameters (HV interval and RV activation time) as factors associated with VA in patients with rTOF. These factors, in addition to Hi-PVC/NSVT, are useful to assess the arrhythmogenic substrate in rTOF patients. The relative contribution of invasive and clinical parameters to the prediction of arrhythmic events in rTOF patients should be addressed in future studies with larger populations. Should our results be replicated, a potential new indication for EPS in patients with rTOF might be revisited. From a clinical point of view, it is more relevant to identify individuals at risk for clinical VA than individuals with induced VA in the EPS. For this reason, we performed regression analyses including only clinical VA as the outcome of interest. Both a pathological HV interval and Hi-PVC/NSVT were significantly associated with VA in the multivariate analysis. Although significant in the univariate analysis, VT inducibility was not confirmed to be independently associated with clinical VA in the multivariate analysis. This could be an effect of the small sample size and the low rate of events in our population, since VT inducibility is a recognized risk factor of VA in rTOF patients.20 Likewise, the RV activation map showed an association with clinical VA in the univariate analysis that was not confirmed in the multivariable model. Future studies in larger series are warranted to elucidate the true role of RV mapping in the prediction of clinical VA in patients with rTOF.
LimitationsOur sample size was small. However, the study provides preliminary data on potential novel parameters associated with VA in patients with rTOF with good discrimination ability, setting the basis for further research, most likely through multicenter studies including larger patient populations. The present study included a selected population of rTOF patients referred for EPS with documented or suspected VT, or prior to pulmonary valve replacement. Therefore, the applicability of our results to the overall population of patients with rTOF should be evaluated in future specific studies. By the time the EPS was performed, 5 patients had undergone a previous ineffective focal ablation. These procedures were performed without navigation and mapping systems and, on the other hand, the approaches were limited to focal ablation that was clinically ineffective and, we believe, had no impact on the RV activation time. Importantly, a sensitivity analysis excluding these patients showed no major changes in the logistic regression models (see ).
CONCLUSIONSA combination of noninvasive and invasive assessment by means of Holter monitoring, EPS, and RV EAM can be useful to assess the risk of VA in patients with rTOF. An HV interval>55ms, RV activation time and Hi-PVC/NSVT are associated with clinical or induced VA in a selected population of rTOF. In addition, an HV interval> 55ms and Hi-PVC/NSVT proved to be related to clinical VA. The findings of our series show that electrophysiological parameters may play a role in the risk stratification of VAs in patients with rTOF.
CONFLICTS OF INTERESTThe Arrhythmia Unit receives fellowship grants from Boston Scientific and research grants from Abbott. N. Rivas-Gándara receives advisory and speaking honoraria from Abbott. J. Francisco-Pascual, B. Benito, J. Perez-Rodon and A. Santos-Ortega receives speaking honoraria from Abbott. The other authors report no conflicts.
I. Ferreira-González is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
Patients with repaired tetralogy Fallot have a high risk of ventricular tachycardia and sudden cardiac death. Risk stratification of ventricular arrhythmias is crucial to apply effective and individualized preventive measures. Recent studies suggest that right ventricle electroanatomical mapping could be useful to identify substrates of higher arrhythmogenic risk.
Invasive electrophysiological study and right ventricle electroanatomical mapping are useful for risk stratification of ventricular arrhythmias in patients with repaired tetralogy of Fallot. An abnormal infra-Hisian conduction (HV interval >55 ms) and longer RV activation time are electrophysiological markers of arrhythmic risk in patients with repaired tetralogy of Fallot.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.12.003