Advances in percutaneous coronary intervention (PCI) have substantially improved outcomes in patients with acute myocardial infarction (AMI). Despite this improvement, heart failure (HF) and ventricular arrhythmias remain common and serious complications of AMI, leading to significant morbidity, mortality, and reduced quality of life. The financial burden of HF on society is already considerable and will further increase with an aging population.1
In clinical practice, therapeutic decisions are usually based on surrogate biomarkers, such as left ventricular ejection fraction (LVEF). However, a significant proportion of patients who die prematurely after AMI have normal or only mildly impaired LVEF. Furthermore, the exact mechanisms of adverse left ventricle (LV) remodelling following AMI are incompletely understood.2 There is a need for imaging modalities to not only demonstrate adverse LV remodelling, but also to identify alternative surrogate biomarkers that could be targeted by treatment after AMI.
Due to its high spatial resolution and ability to characterize tissue composition, cardiovascular magnetic resonance (CMR) imaging provides markers of myocardial damage (such as infarct size [IS], myocardial salvage, and microvascular obstruction [MVO]) with established incremental prognostic value to standard clinical, electrocardiographic and functional biomarkers, including LVEF.3 This review provides an overview of established and novel prognostic CMR markers and their potential role in the risk stratification of adverse cardiovascular events and the assessment of HF treatment strategies in AMI survivors.
CMR ASSESSMENT OF LVEFThe intense inflammatory response occurring after AMI leads to tissue necrosis, scar formation, and a subsequent reduction in contractile function in the area of infarction. Consequently, LV contraction becomes asymmetrical, meaning wall tension is no longer homogeneously distributed in the LV. This imbalance can lead to LV cavity dilatation, causing an increase in LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV).4
The term “adverse LV remodelling” is applied when the heart is unable to maintain its geometry following AMI, resulting in a >20% rise in LVEDV and a >15% rise in LVESV. Thus, traditional targets for clinical trials have involved assessment of LV volumes and LVEF following AMI. This began with ventriculography techniques by White et al.5 in 1987, and was followed by echocardiography-based studies.3,4 Today, CMR is recognized as the most accurate tool for assessing LV function and LVEF and is therefore increasingly used as an endpoint in clinical trials. CMR-based studies in AMI survivors have also established LVEF as an independent risk factor for predicting future major adverse cardiac events (MACE).6
Advanced imaging of global and regional myocardial deformation, also referred to as strain imaging, holds promise. Recent evidence suggests that global longitudinal impairment provides independent and incremental prognostic information for the prediction of all-cause mortality, as shown by Eitel et al.7 in 1235 patients within the first 10 days after AMI. During a 12-month follow-up period, global longitudinal strain (GLS) impairment in particular was found to be independently associated with MACE, even after adjustments were made for established CMR markers of poor prognosis such as LVEF and MVO. The value of GLS in predicting MACE postmyocardial infarction (MI) may relate to the subendocardial location of the longitudinal fibres, the area most affected by AMI.4 When combined with LVEF and IS, GLS assessments provided incremental prognostic value.
Romano et al.8 also evaluated the prognostic value of CMR feature tracking (FT)-derived GLS in patients with ischemic and nonischemic cardiomyopathy. In a large observational multicenter study, 1012 patients with ejection fraction (EF)<50% had interpretable CMR scans. During a median follow-up of 4.4 years, even after adjustment for clinical and imaging risk factors (age, body mass index, diabetes, hyperlipidaemia, LV end-diastolic volume index, late gadolinium enhancement [LGE] extent, EF), GLS remained a significant independent predictor of death.
Current clinical guidelines recommend implantable cardioverter device (ICD) placement in patients with an LVEF <35%. In the study by Romano et al., patients with a relatively preserved GLS had very few adverse events, irrespective of whether their EF was above or below 35%. This raises the question of whether GLS could play a future role in the risk stratification of patients with ischemic or nonischemic cardiomyopathy, but further study is required in this field.
CMR TISSUE CHARACTERIZATIONInfarct Size AssessmentLGE has become the criterion standard for delineating irreversibly injured myocardium, and hence calculating IS. This is typically expressed as a percentage of the total LV mass. Characterizing necrotic tissue shortly after AMI can be challenging, however, as it can be difficult to distinguish scar from edema, especially in the first 72hours.9
In a recent meta-analysis by Stone et al.,10 2632 patients were pooled from 10 randomized primary PCI trials. The patients had undergone either CMR (n=1887) or single-photon emission computed tomography (n=743) assessment for IS within 1 month of AMI. After multivariate analysis, IS was found to be a strong independent predictor of all-cause mortality and HF hospitalization within 1 year (P <.0001 for both). To date, this is the largest and most robust examination of the relationship between IS and prognosis after reperfusion therapy and confirms findings from previous smaller studies.
One of the major advantages of CMR is the ability to acquire 3-dimensional images, allowing accurate visualization of the right ventricle (RV). Among 450 STEMI patients analyzed by Grothoff et al.,11 the detection of RV infarction on CMR was a strong and independent predictor of MACE.
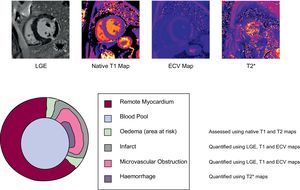
Myocardial SalvageBy comparing the “area at risk” assessed as edematous myocardium postischemic insult and the amount of scarred tissue, the amount of salvaged myocardium can be calculated and expressed as the myocardial salvage index (Figure 1).
As well as IS, the amount of salvaged myocardium has prognostic significance. Eitel et al.12 performed CMR in 208 consecutive reperfused AMI patients within 4 days of their index event. In this cohort, MACE rates were significantly lower among patients with the highest myocardial salvage index and were inversely correlated with MACE and mortality at 6 months of follow-up. This finding has been supported by more recent studies.
Microvascular ObstructionDespite the restoration of the patency of epicardial coronary circulation, a variety of mechanisms, including distal embolization of thrombus/plaque, vasospasm, and reperfusion-associated injuries, lead to the persistence of hypoperfusion of myocardial tissue in a considerable number of patients after PCI.13 This phenomenon is referred to as MVO. With CMR, MVO is detected by the lack of gadolinium uptake within hyperenhanced areas. Some authors have postulated that MVO limits the delivery of endogenous promoters of postinfarction remodelling, predisposing patients to HF and arrhythmias.
Several studies have demonstrated a prognostic significance of the presence of MVO following AMI. Waha et al.13 recently pooled data from 1688 patients recruited to 7 randomized primary PCI trials who underwent CMR assessment within 7 days. They found that patients with MVO had larger IS than those without. A graded response was noted between MVO and all-cause mortality and HF hospitalizations within 1 year. On multivariable analyses adjusting for both MVO and IS, MVO remained significantly associated with all-cause mortality (P <.0001), but not with HF hospitalization, whereas IS was an independent predictor of both. Conversely the absence of MVO was an independent predictor of LV recovery, reflected in improvements of LVEF over time.
Intramyocardial HemorrhageSevere microvascular injury causes a loss of endothelial integrity, leading to intramyocardial hemorrhage (IMH). This eventually leads to iron deposition in the myocardium, whose persistence provides a source of prolonged inflammatory burden in the convalescent phase, promoting adverse LV remodelling. In an animal study by Cokic et al.,14 higher iron content within infarcted canine hearts was associated with a significant prolongation of the QT and QTc interval, a phenomenon typically associated with ventricular arrhythmias. Observations from this study suggest that the extent of iron deposition could be a predictor of arrhythmias in humans.
The breakdown products of hemoglobin alter the magnetic properties by shortening the T2* relaxation times. As a consequence, CMR T2* tissue characterization techniques offer the possibility of depicting and quantifying the amount of IMH and persistent iron.1
The predictive relevance of LV remodelling with IMH quantification by CMR was shown by Carberry et al.15 in a prospective study of 203 AMI survivors following reperfusion. These authors demonstrated that patients with IMH at 6 months had higher LVEDV and worse LVEF. In addition, there was a 4-fold increase in all-cause mortality and HF and a 3-fold increase in the likelihood of MACE. This builds on the evidence of previous smaller studies and leads to the conclusion that persistence of iron defines a high-risk group among AMI survivors.
Native T1, T2 and Extracellular VolumeAlthough LGE is the criterion standard for scar analysis, it only allows a dichotomous assessment of the presence or absence of scar tissue, but not of the severity of pathophysiological changes within the scar. Furthermore, LGE analysis is signal intensity/threshold-based and hence strictly dependent on the methodology and execution of the scan (ie, acquiring images with appropriate time delay after contrast injection in order to “null” the myocardium) and postprocessing (ie, choosing an appropriate reference “region of interest” for the remote myocardium).
Native T1 values are determined by how rapidly proton spins re-equilibrate their longitudinal magnetization after being excited by a radiofrequency pulse. T1 mapping refers to pixel-wise measurements of absolute T1 relaxation times on a quantitative map.
In contrast, T2 values are determined by the speed at which proton spins re-equilibrate their transverse magnetization following the radiofrequency pulse. T2-weighted imaging enables the visualization of myocardial edema and is able to identify acute or recent myocardial ischemic injury.
Native T1 and T2 mapping techniques offer the possibility of accurately assessing edematous changes from a recent infarction and the severity of ischemic injury. Voxel-wise quantitative assessment of the tissue composition allows determination of the severity of damage on a continuous scale in addition to volume quantification. The predictive relevance of alterations in T1 and T2 values has been demonstrated in several studies. For example, Carrick et al.16 performed CMR scans on 300 reperfused AMI survivors on day 2 and 6 months after the index event. They were able to demonstrate that native T1 values within the infarct core were inversely associated with adverse remodelling, all-cause mortality, and HF-related hospital admissions within a 2.5 year follow-up period.
Postcontrast T1 mapping techniques are also available and, combined with native T1, are used to assess extracellular volumes (ECV). Following intravenous administration, currently used contrast agents distribute only into the extracellular space and shorten T1 relaxation times of myocardium in proportion to the local concentration of contrast agent. Therefore, in fibrotic and scar tissue where the extracellular space is expanded, postcontrast T1 relaxation times will be shorter than in areas with healthy myocardium. Kidambi et al.17 performed acute (day 2) and convalescent (3 month) CMR scans in 99 patients following AMI, and showed that acute infarct ECV was also a predictor of adverse regional and global LV recovery.
Remote MyocardiumStructural changes in LV remodelling are not limited to the territory of the culprit artery, but also affect remote myocardium. In the weeks following AMI, the remodelling process is predominantly driven by hypertrophy of the healthy remote myocardium for compensatory purposes. This phenomenon has been previously demonstrated in histological animal studies, but CMR allows a more comprehensive in vivo evaluation of such changes in humans with prognostic implications.
Carberry et al.18 performed CMR scans on 140 patients, on day 2 and 6 months post-AMI. Using multivariate regression, they demonstrated that LVEF was inversely associated with remote zone ECV alterations (P <.001).
In a more recent study, Reinstadler et al.19 performed noncontrast T1 mapping in 255 AMI patients following reperfusion. These authors were able to demonstrate that patients with increased remote zone native T1 had significantly higher MACE at 6 months. The inclusion of remote zone T1 alterations to more conventional risk factors such as LVEF and IS added incremental prognostic information.
PROGNOSTIC RISK STRATIFICATION: THE FUTURE?As shown, there is an abundance of CMR techniques available for prognostic risk stratification in AMI survivors. Increasingly, CMR parameters are used as surrogate endpoints of outcome in clinical trials, for example to compare the efficacy of reperfusion strategies. The attraction of surrogate endpoints compared with clinical endpoints (such as death, recurrent AMI, or HF related hospital admissions) is that they allow studies with smaller sample sizes and shorter follow-up times. In clinical practice, however, more basic measurements such as LVEF and Killip class scores for HF continue to influence clinical management decisions due to a perceived lack of sufficient evidence and the impracticalities of CMR scanning in the acute phase following AMI.
Use of CMR in Heart FailureDespite advances in PCI, complementary reperfusion therapies designed to prevent HF have thus disappointed in clinical trials.9 In a review of such therapies by Hausenloy et al.,20 the authors recommend specifically targeting high-risk patients who have more to gain, such as patients with larger IS and MVO, in future clinical trials. The CMR risk scores proposed by Pontone et al.21 for example could feasibly be used for this purpose.
Limitations of Current Studies and Clinical TrialsAs discussed above, CMR markers such as IS and MVO are stronger predictors of clinical outcomes than LVEF and volumes. However, calculating the acute MI size is heavily influenced by the timing of the scan following AMI, the dose and type of contrast agent used, the timing of LGE image acquisition following contrast administration, as well as the method used for quantification. Therefore, there is a need to standardize the protocols of CMR scans shortly after AMI.
Bulluck et al.9 conducted a review of 62 randomized controlled trials (RCTs) that used CMR to assess MI size after AMI, comparing methods and results. They found that there was significant heterogeneity in the scanning protocols between the different trials, hampering fair interpretation and study comparisons. Based on their findings, the authors discuss and make recommendations (Table 1) for more standardized acute CMR scan protocols in the future. This would pave the way for more collaborative research between different CMR centers, leading to more robust clinical trials. It would also facilitate the integration of acute CMR scans into clinical practice.
Recommendations for CMR Protocols in Future Trials
| Category | Recommendation |
|---|---|
| Optimal timing of acute scans24 | Because of excess edema in the early phase, LGE analysis from CMR scans performed before 3 d following AMI overestimates MI size.9,25 MVO and IMH size peaks on day 3 after AMI before gradually reducing in size.26 The optimal time for the acute CMR scan it thought to be within 3-5 d following AMI. |
| Optimal timing of follow-up scans9 | Pairing acute and follow-up scans allow the study of LV remodelling. Chronic MI size is relatively stable between 1 mo and 1 y.27 Follow-up scans at 6 mo is the most popular interval in clinical trials. |
| Timing of LGE acquisitions9 | Early acquisition (< 8 min) of LGE images results in overestimation of MI size. Acquiring at 25 mins produces the most accurate estimate. As a compromise between scan times and accuracy, it is recommended to acquire LGE images between 10 and 15min.9 |
| Methods of quantifying MI size28 | Manual contouring is time-consuming but remains the criterion standard. Semiautomated models including 5-SD and “full width half method” techniques are more reproducible and time-saving but still require manual input. Thus far, the accuracy of semiautomated methods for infarct sizing has proved inferior to manual contouring. A more stringent 6-SD model may be more reliable and accurate but needs further development. |
AMI, acute myocardial infarction; CMR, cardiovascular magnetic resonance; IMH, intramyocardial hemorrhage; LGE, late gadolinium enhancement; LV, left ventricle; MI, myocardial infarction; MVO, microvascular obstruction; SD, standard deviation.
A limitation of CMR in the context of AMI is the relatively long scan times, which can be up to an hour for comprehensive protocols. This can be difficult for patients with large infarcts and poor LV function; shortening of scan times is required to make CMR more feasible and practical in AMI and HF patients. Contrast agents can be contraindicated in some patients with advanced renal disease or contrast allergies and noncontrast methods, such as parametric mapping, warrant further development in these patient groups. Free-breathing techniques involving T2/T2*-weighted images have shown potential in saving time. Simultaneous T1/T2/proton density acquisition using magnetic resonance fingerprinting is a further avenue that is being explored to make CMR scanning more efficient.
Using CMR to Select Patients for Implantable Cardioverter DefibrillatorsCurrent clinical guidelines recommend an ICD for primary prevention in patients with symptomatic HF and LVEF <35%, 40 days after their AMI. However, mortality rates are at their highest within the first 30 days post-MI, a quarter of which are due to ventricular arrhythmias, and yet currently there are no risk stratification tools in this acute phase after AMI.
An acute MI size of >31% of LV, in combination with LVEF <35% by CMR within the first week post-AMI, has been shown to predict adverse arrhythmic cardiac events at 2 years, according to a study by Izquierdo et al.22 involving 440 AMI patients. Jablonowski et al.23 studied a cohort of patients who underwent ICD implantation for primary prevention. They performed a retrospective analysis on CMR scans prior to ICD implantation, and through evaluating LGE border zone patterns, were able to predict which patients would require appropriate ICD therapy. These studies demonstrate that CMR has the potential to contribute to early risk stratification and guide ICD therapy in AMI survivors, but further evidence is needed to determine a role for CMR in clinical practice.
Targets for Future CMR TrialsTo incorporate the use of CMR biomarkers more into clinical practice, future CMR-based clinical trials should seek to compare the different CMR techniques with the goal of proving their superiority at predicting arrhythmias and MACE over traditional markers such as LVEF and symptomatic HF after 40 days. For example, Pontone at al.21 directly compared CMR and transthoracic echocardiography. Their model of using CMR-LVEF ≤ 35% plus LGE detection predicted MACE and ventricular arrhythmia more accurately than transthoracic echocardiography-LVEF.
CONCLUSIONCMR has emerged as a robust imaging technique for AMI patient prognostic stratification. It provides numerous imaging biomarkers for the prediction of LV remodelling, MACE, and ventricular arrhythmias. As such, it is an ideal modality for the provision of surrogate endpoints for large clinical trials. CMR holds the potential to play an even larger role in clinical practice if scans can be made quicker, safer, and more feasible.
FUNDINGE. Dall’Armellina is a British Heart Foundation Intermediate Clinical Research Fellow (FS/13/71/30378). S. Plein is funded by a British Heart Foundation Chair (CH/16/2/32089).
CONFLICTS OF INTERESTNone declared.