Data are scarce on outcomes of transvenous lead removal (TLR) in adult congenital heart disease (CHD). We evaluated the safety of the TLR procedure in adult CHD patients from a 10-year national database.
MethodsWe used the Healthcare Cost and Utilization Project Nationwide Inpatient Sample to identify TLR procedures in adult patients with and without CHD from 2005 to 2014. Outcomes included in-hospital mortality and complications.
ResultsOf 132 068 adult patients undergoing TLR, 1939 had simple CHD, 657 had complex CHD, and 626 had unclassified CHD. The number of TLR procedures in adult CHD slightly increased from 236 in 2005 to 445 in 2014, with fluctuations over the study period. The overall rate of any complications in the TLR procedure was 16.6% in patients with CHD vs 10.1% in patients without CHD (P <.001). In a propensity score-matched cohort, CHD was associated with a higher risk of any complication after full adjustment vs patients without CHD (adjusted odd ratio, 1.49; 95% confidence interval, 1.11-1.99; P=.007). Simple and complex CHD were associated with 1.5- and 2.1-fold increased risks of any TLR-related complication, respectively. CHD was not associated with an increased risk of in-hospital mortality (adjusted odd ratio, 0.77; 95% confidence interval, 0.42-1.39; P=.386).
ConclusionsCompared with patients without CHD, adult patients with simple and complex CHD undergoing TLR are more likely to have complications but show no increase in mortality.
Keywords
Congenital heart disease (CHD) is the most prevalent birth defect in the United States, affecting approximately 0.4% to 1% of live births.1–3 The survival of individuals with CHD has markedly increased with improved surgical, medical, and interventional care.4,5 Consequently, there is a growing population of adult patients with CHD, as well as that of patients with complex CHD.6,7 As highlighted by the Adult Congenital Heart Association, more limited evidence is available regarding the adult CHD population compared with the neonatal and childhood CHD population.8
The significant burden of cardiac arrhythmias in CHD patients caused by the congenital defect itself or its surgical repair9 creates unique challenges for electrophysiological procedures.10 The adult CHD population increasingly requires pacemakers and implantable cardioverter-defibrillators.11 As more cardiac devices are implanted and more lead failures occur in CHD patients,12 transvenous lead removal (TLR) in these patients becomes more and more inevitable. The outcomes of TLR in CHD need to be understood for treatment-planning. However, little is known about the performance and safety of TLR in CHD patients in the United States. Although several case studies have investigated this topic, the results are mostly confined to children or young adults and are compromised by the limited case number.13–18 Only 2 studies reported results in adult CHD, involving 16 and 22 patients, respectively, with their results indicating a higher major complication rate and lower successful extraction rate in such patients.13,14 In the present study, we evaluated the outcomes of the TLR procedure in adult CHD patients by using a nationally representative database of hospital admissions in the United States from 2005 to 2014.
METHODSWe conducted a cross-sectional analysis of hospital discharge information pertaining to 2005 to 2014 from administrative files pertaining to the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS).19 The NIS is the largest, publicly available, all-payer inpatient database in the United States.20 It comprises discharge-level data on about 8 million hospitalizations per year and approximates a stratified sample of 20% of inpatient admissions in the United States. Statistical sampling weights provided by the NIS permit inferences for a nationally representative population21 and have been validated against other hospital registries.22 Each record in the NIS includes clinical and resource use information, with all procedural and diagnostic International Classification of Diseases (ICD) codes recorded for each patient's hospital discharge. We leveraged this comprehensive, nationwide database to identify complications and the in-hospital mortality of TLR in patients with and without CHD.
The following ICD-9 procedure codes were used to select patients who underwent TLR: 37.77, 37.79, 37.89, and 37.99.23–26 Only patients 18 years of age and older were included in the study. To avoid potential confounding of complications, we excluded patients who underwent other invasive procedures during their stay, as well as those missing values on age, sex, and mortality, giving a total of 27 347 records.
CHD patients were categorized as simple, complex, or unclassified based on the 32nd Bethesda Conference report.27 The list of the categories, along with their ICD-9 codes, is shown in , adapted from a previous publication.11 Patients with simple CHD with coexisting complex lesions or pulmonary hypertension were categorized as complex according to the recommendations of the Bethesda classification.27
In the present study, independent demographic variables included age (grouped as 18-34, 35-49, 50-64, 65-79, and ≥ 80 years), sex, ethnicity (White, Black, Hispanic, other, unknown), median household income based on patient zip code (quartiles), primary payer (Medicare, Medicaid, private, self-pay/no charge/other), and admission type (elective, nonelective). We defined comorbid condition severity by using the Deyo modification of the Charlson Comorbidity Index.28 This index uses 17 comorbid conditions with differential weighting and total scores ranging from 0 to 33, with higher scores representing greater comorbidity burden. Independent hospital-provider variables included hospital bed size (small, medium, or large), hospital region (Northeast, Midwest, South, or West), location (urban or rural), and teaching status (teaching or nonteaching). Hospital volumes for TLR were defined on a year-to-year basis according to European Heart Rhythm Association criteria (low-volume center, <15 procedures/y; medium-volume center, 15-30 procedures/y; high-volume center, ≥ 30 procedures/y).29,30 We also divided the study into two 5-year periods (2005-2009 and 2010-2014).
Primary outcomes included in-hospital mortality and complications in the setting of TLR procedures. Procedural complications were based on previous literature regarding TLR complications.23,31 They were identified by their ICD-9 codes () and categorized as vascular injury, hemorrhage (requiring a transfusion or not), pericardial complications (hemopericardium, cardiac tamponade, pericardiocentesis, pericardiotomy), pneumothorax or hemothorax (requiring a chest tube or not), iatrogenic cerebrovascular infarction or hemorrhage, acute renal failure requiring new hemodialysis, and need for heart and pericardium repair. Any complication was defined as the occurrence of ≥ 1 of the postprocedural complications listed.
Demographic and clinical characteristics were compared by chi-square test for categorical variables. Fisher exact test was used where appropriate. The sampling weights and clustering were considered in the analysis. We used sampling weights provided by the NIS to estimate the number of TLRs in CHD patients over time on a national level. Two-level hierarchical models were created to determine the adjusted relative risks of in-hospital mortality and complications, with the unique hospital identification number incorporated as a random effect within the model. Results are reported as odds ratios (ORs) with 95% confidence intervals (95%CIs). We first performed the regression analysis in model 1 (unadjusted model), followed by adjustment for all of the independent variables included in the study in model 2. In model 3, we further adjusted for device infection. Because race was missing for 16.3% of the population, we did not include it in the model. To account for differences in baseline characteristics, we used propensity score matching between patients with and without CHD. The propensity score was estimated for each patient using a logistic regression model including 13 baseline variables. We applied the propensity score to pair each CHD patient with 2 individuals without CHD by using the nearest neighbor method with a caliper of 0.25 and no replacement. Matching quality was assessed by absolute standardized differences, with a value <0.1 considered not significant. Statistical analyses were performed using Stata/SE 14.0 (StataCorp, College Station, United States) and SPSS 23.0 (IBM Corporation, Chicago, United States). Two-tailed P <.05 was considered significant.
Patient information was anonymized before analysis, and certification to use these deidentified HCUP data was obtained from the University of California (San Francisco) Committee on Human Research.
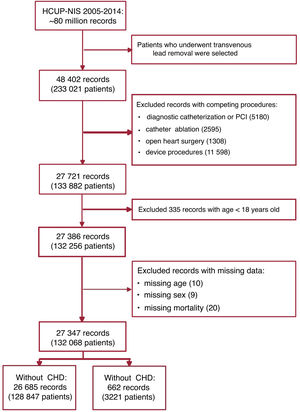
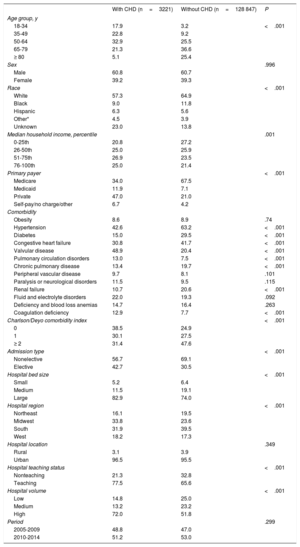
RESULTSWe identified 233 021 patients who underwent TLR from 2005 to 2014. The cohort selection flowchart is shown in figure 1. Patients who underwent other procedures were excluded, including diagnostic catheterization or percutaneous coronary intervention (n=5180), catheter ablation (n=2595), open-heart surgery (n=1308), and other device procedures (n=11 598). We also excluded records with missing values on age (n=10), sex (n=9), and mortality (n=20). The final study cohort comprised 132 068 patients; 3221 (2.4%) were CHD patients. Demographic and hospital characteristics varied between patients with and without CHD, all of whom underwent TLR procedures (table 1). The CHD group contained higher percentages of individuals 18 to 34 and 35 to 49 years old. There was no difference in sex between the 2 groups. CHD patients were less likely to be White and to live in the lowest income zip code. The CHD group showed a much lower proportion of Medicare as primary payer (34.0% vs 67.5%). Patients with CHD had a lower proportion of severe comorbidities (a Charlson/Deyo Index ≥ 2; 31.4% vs 47.6%) and were more likely to be electively admitted (42.7% vs 30.5%) than those without CHD. In total, 77.5% of the CHD patients underwent TLR procedures in a teaching hospital vs 65.6% in the non-CHD group (P <.001). CHD patients who underwent a TLR procedure were more likely to be admitted to hospitals with a large bed size and high volume (both P <.001).
Cohort selection flowchart. Records represent the number of individuals in the database and patients represent the number of individuals nationally estimated by weights. CHD, congenital heart disease; HCUP-NIS, Healthcare Cost and Utilization Project Nationwide Inpatient Sample; PCI, percutaneous coronary intervention.
Demographic, clinical, and hospital characteristics of adult patients with and without congenital heart disease undergoing transvenous lead removal in the United States from 2005 to 2014
| With CHD (n=3221) | Without CHD (n=128 847) | P | |
|---|---|---|---|
| Age group, y | |||
| 18-34 | 17.9 | 3.2 | <.001 |
| 35-49 | 22.8 | 9.2 | |
| 50-64 | 32.9 | 25.5 | |
| 65-79 | 21.3 | 36.6 | |
| ≥ 80 | 5.1 | 25.4 | |
| Sex | .996 | ||
| Male | 60.8 | 60.7 | |
| Female | 39.2 | 39.3 | |
| Race | <.001 | ||
| White | 57.3 | 64.9 | |
| Black | 9.0 | 11.8 | |
| Hispanic | 6.3 | 5.6 | |
| Other* | 4.5 | 3.9 | |
| Unknown | 23.0 | 13.8 | |
| Median household income, percentile | .001 | ||
| 0-25th | 20.8 | 27.2 | |
| 26-50th | 25.0 | 25.9 | |
| 51-75th | 26.9 | 23.5 | |
| 76-100th | 25.0 | 21.4 | |
| Primary payer | <.001 | ||
| Medicare | 34.0 | 67.5 | |
| Medicaid | 11.9 | 7.1 | |
| Private | 47.0 | 21.0 | |
| Self-pay/no charge/other | 6.7 | 4.2 | |
| Comorbidity | |||
| Obesity | 8.6 | 8.9 | .74 |
| Hypertension | 42.6 | 63.2 | <.001 |
| Diabetes | 15.0 | 29.5 | <.001 |
| Congestive heart failure | 30.8 | 41.7 | <.001 |
| Valvular disease | 48.9 | 20.4 | <.001 |
| Pulmonary circulation disorders | 13.0 | 7.5 | <.001 |
| Chronic pulmonary disease | 13.4 | 19.7 | <.001 |
| Peripheral vascular disease | 9.7 | 8.1 | .101 |
| Paralysis or neurological disorders | 11.5 | 9.5 | .115 |
| Renal failure | 10.7 | 20.6 | <.001 |
| Fluid and electrolyte disorders | 22.0 | 19.3 | .092 |
| Deficiency and blood loss anemias | 14.7 | 16.4 | .263 |
| Coagulation deficiency | 12.9 | 7.7 | <.001 |
| Charlson/Deyo comorbidity index | <.001 | ||
| 0 | 38.5 | 24.9 | |
| 1 | 30.1 | 27.5 | |
| ≥ 2 | 31.4 | 47.6 | |
| Admission type | <.001 | ||
| Nonelective | 56.7 | 69.1 | |
| Elective | 42.7 | 30.5 | |
| Hospital bed size | <.001 | ||
| Small | 5.2 | 6.4 | |
| Medium | 11.5 | 19.1 | |
| Large | 82.9 | 74.0 | |
| Hospital region | <.001 | ||
| Northeast | 16.1 | 19.5 | |
| Midwest | 33.8 | 23.6 | |
| South | 31.9 | 39.5 | |
| West | 18.2 | 17.3 | |
| Hospital location | .349 | ||
| Rural | 3.1 | 3.9 | |
| Urban | 96.5 | 95.5 | |
| Hospital teaching status | <.001 | ||
| Nonteaching | 21.3 | 32.8 | |
| Teaching | 77.5 | 65.6 | |
| Hospital volume | <.001 | ||
| Low | 14.8 | 25.0 | |
| Medium | 13.2 | 23.2 | |
| High | 72.0 | 51.8 | |
| Period | .299 | ||
| 2005-2009 | 48.8 | 47.0 | |
| 2010-2014 | 51.2 | 53.0 | |
CHD, congenital heart disease.
Values are presented as %. Percentages may not sum to 100% due to missing data. Overall, missing data comprised <4% except for race, which had a 14% proportion of missing data.
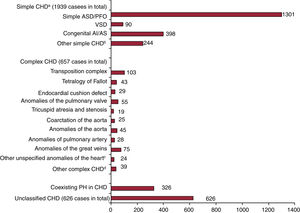
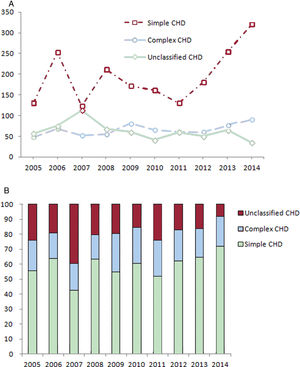
As shown in figure 2, we identified 1939 patients with simple CHD, 657 with complex CHD, and 626 with unclassified CHD who underwent a TLR procedure between 2005 and 2014. A simple atrial septal defect or patent foramen ovale were the most common congenital defects overall. Congenital aortic insufficiency and stenosis were also common simple CHDs. Of complex congenital defects, transposition complex and anomalies of the great veins were the most common. In total, 326 patients had coexisting pulmonary hypertension at admission. The number of TLR procedures in complex CHD slightly climbed from 48 in 2005 to 90 in 2014, with fluctuations over the study period (figure 3A). TLR procedures in simple CHD patients increased from 131 in 2005 to 320 in 2014, with sharp growth after 2011. The proportions of the 3 types of CHD by year are presented in figure 3B.
Types of congenital heart disease in patients undergoing transvenous lead removal. AI, aortic insufficiency; AS, aortic stenosis; ASD, atrial septal defect; CHD, congenital heart disease; PFO, patent foramen ovale; PH, pulmonary hypertension; VSD, ventricular septal defect. aDid not account for patients with coexisting pulmonary hypertension. bIncluding congenital mitral stenosis, congenital mitral insufficiency, coronary artery anomaly, other bulbus cordis anomaly or septal defect, unspecified defect of septal closure, and congenital heart block. cIncluding subaortic stenosis, cor triatriatum, infundibular pulmonic stenosis, and obstructive anomalies of the heart (NEC). dIncluding common truncus, common ventricle, cor biloculare, hypoplastic left heart syndrome, patent ductus arteriosus, and Ebstein's anomaly.
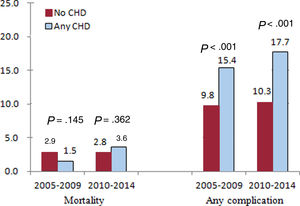
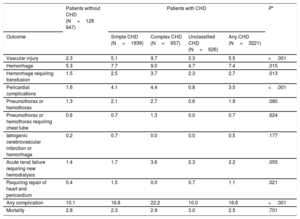
As shown in table 2, the overall complication rates in the TLR procedure were 16.6% in patients with CHD and 10.1% in patients without CHD (P <.001). There was no significant difference in the total in-hospital mortality rate between the 2 groups. In CHD patients, the most common complications included any type of hemorrhage not requiring blood transfusion (7.4%), followed by vascular injury (5.5%), pericardial complications (3.5%), hemorrhage requiring blood transfusion (2.7%), and acute renal failure requiring new hemodialysis (2.2%). Complication rates were consistently higher in CHD patients than in non-CHD patients both from 2005 to 2009 and from 2009 to 2014 (figure 4).
Complication and mortality rates of transvenous lead removal in patients with and without congenital heart disease
| Patients without CHD (N=128 847) | Patients with CHD | P* | ||||
|---|---|---|---|---|---|---|
| Outcome | Simple CHD (N=1939) | Complex CHD (N=657) | Unclassified CHD (N=626) | Any CHD (N=3221) | ||
| Vascular injury | 2.3 | 5.1 | 9.7 | 2.3 | 5.5 | <.001 |
| Hemorrhage | 5.3 | 7.7 | 9.0 | 4.7 | 7.4 | .015 |
| Hemorrhage requiring transfusion | 1.5 | 2.5 | 3.7 | 2.3 | 2.7 | .013 |
| Pericardial complications | 1.6 | 4.1 | 4.4 | 0.8 | 3.5 | <.001 |
| Pneumothorax or hemothorax | 1.3 | 2.1 | 2.7 | 0.6 | 1.9 | .080 |
| Pneumothorax or hemothorax requiring chest tube | 0.6 | 0.7 | 1.3 | 0.0 | 0.7 | .624 |
| Iatrogenic cerebrovascular infarction or hemorrhage | 0.2 | 0.7 | 0.0 | 0.0 | 0.5 | .177 |
| Acute renal failure requiring new hemodialysis | 1.4 | 1.7 | 3.6 | 2.3 | 2.2 | .055 |
| Requiring repair of heart and pericardium | 0.4 | 1.5 | 0.0 | 0.7 | 1.1 | .021 |
| Any complication | 10.1 | 16.8 | 22.2 | 10.0 | 16.6 | <.001 |
| Mortality | 2.8 | 2.3 | 2.9 | 3.0 | 2.5 | .701 |
CHD, congenital heart disease.
Any complication was identified as the occurrence of at least one of vascular injury, hemorrhage, pericardial complications, pneumothorax or hemothorax, iatrogenic cerebrovascular infarction or hemorrhage, acute renal failure requiring new hemodialysis, or need for heart or pericardium repair.
N represents the number of individuals nationally estimated by weights.
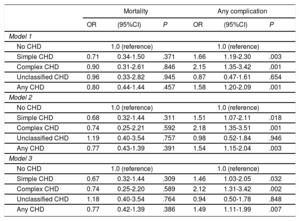
Propensity score matching yielded 1981 patients: 662 in the CHD group and 1319 in the non-CHD group. Baseline characteristics were equally distributed between the 2 study groups after matching (). Table 3 shows regression analyses concerning the risk of TLR-related mortality and complications in patients with and without CHD in the matched cohort. After adjustment for all independent demographic and clinical variables included in the study, CHD was not associated with increased risk of in-hospital mortality (adjusted OR [aOR], 0.77; 95%CI, 0.42-1.39; P=.386). When the 3 types of CHD were analyzed separately, none was associated with an increased risk of mortality in any of the models. CHD was associated with a higher risk of any complication even after adjustment for device infection (aOR, 1.49; 95%CI, 1.11-1.99; P=.007). Specifically, simple and complex CHD were associated with around 1.5-fold and 2.1-fold increased risks of any TLR-related complication, respectively (simple CHD: aOR, 1.46; 95%CI, 1.03-2.05; P=.032; complex CHD: aOR, 2.12; 95%CI, 1.31-3.42; P=.002). The regression results were similar in the total population (). Considering the large proportion of unclassified CHD patients, we performed sensitivity analyses that excluded these patients. The results were also consistent for patients with and without CHD in both the matched cohort and total population after full adjustment (matched cohort: aOR, 0.72; 95%CI, 0.37-1.39; P=.323 for mortality; and aOR, 1.63; 95%CI, 1.20-2.22; P=.002 for any complication; total population: aOR, 1.08; 95%CI, 0.61-1.91; P=.801 for mortality; and aOR, 1.66; 95%CI, 1.30-2.11; P <.001 for any complication).
In-hospital mortality and complications related to transvenous lead removal in patients with congenital heart disease vs those without after propensity score matching
| Mortality | Any complication | |||||
|---|---|---|---|---|---|---|
| OR | (95%CI) | P | OR | (95%CI) | P | |
| Model 1 | ||||||
| No CHD | 1.0 (reference) | 1.0 (reference) | ||||
| Simple CHD | 0.71 | 0.34-1.50 | .371 | 1.66 | 1.19-2.30 | .003 |
| Complex CHD | 0.90 | 0.31-2.61 | .846 | 2.15 | 1.35-3.42 | .001 |
| Unclassified CHD | 0.96 | 0.33-2.82 | .945 | 0.87 | 0.47-1.61 | .654 |
| Any CHD | 0.80 | 0.44-1.44 | .457 | 1.58 | 1.20-2.09 | .001 |
| Model 2 | ||||||
| No CHD | 1.0 (reference) | 1.0 (reference) | ||||
| Simple CHD | 0.68 | 0.32-1.44 | .311 | 1.51 | 1.07-2.11 | .018 |
| Complex CHD | 0.74 | 0.25-2.21 | .592 | 2.18 | 1.35-3.51 | .001 |
| Unclassified CHD | 1.19 | 0.40-3.54 | .757 | 0.98 | 0.52-1.84 | .946 |
| Any CHD | 0.77 | 0.43-1.39 | .391 | 1.54 | 1.15-2.04 | .003 |
| Model 3 | ||||||
| No CHD | 1.0 (reference) | 1.0 (reference) | ||||
| Simple CHD | 0.67 | 0.32-1.44 | .309 | 1.46 | 1.03-2.05 | .032 |
| Complex CHD | 0.74 | 0.25-2.20 | .589 | 2.12 | 1.31-3.42 | .002 |
| Unclassified CHD | 1.18 | 0.40-3.54 | .764 | 0.94 | 0.50-1.78 | .848 |
| Any CHD | 0.77 | 0.42-1.39 | .386 | 1.49 | 1.11-1.99 | .007 |
95%CI, 95% confidence interval; CHD, congenital heart disease; OR, odds ratio.
Model 1: unadjusted model.
Model 2: adjusted for age, sex, household income, primary payer, Charlson/Deyo Comorbidity Index, admission type, hospital bed size, hospital region, hospital location, hospital teaching status, hospital volume, and period.
Model 3: adjusted for model 2 and device infection.
Any CHD and the 3 types of CHD were analyzed in 2 separate regression processes with no CHD as the reference.
Using a national all-payer database of hospital discharges in the United States, we obtained the following primary findings: a) TLR procedures were more commonly performed in simple CHD patients (∼50%) than in those with complex and unclassified types, with a slight growth in the number of TLR procedures in CHD patients from 2005 to 2014; b) CHD was associated with higher risk of TLR-related complications after adjustment for potential confounders, with simple and complex CHD associated with about 1.5-fold and 2.1-fold higher risks of TLR procedural complications, respectively, compared with non-CHD patients; and c) in-hospital mortality after TLR was similar in adult patients with and without CHD. To our knowledge, our study is the first to report a nationally representative experience of TLR in the adult CHD population, providing real-world data beyond the experience of a few specialized referral centers.
In the present study, patients with CHD who underwent TLR procedures were generally younger and had fewer comorbidities, which is consistent with other device implantation procedures.32 Unexpectedly, the number of TLR procedures performed in adult CHD patients did not significantly increase from 2005 to 2014, despite advances in lead extraction tools and techniques, such as laser and telescoping sheaths.31,33 Although there was a visible increase in TLR in simple CHD patients after 2011, the total estimated number of cases was still less than 500 in 2014 throughout the United States. This finding suggests that electrophysiologists remain hesitant to perform TLR in CHD patients, possibly because there is limited information on procedural outcomes in the literature.
There is scant population-based information on the safety of TLR procedures in CHD patients because studies on CHD admissions have tended to describe major common diseases or procedures, such as heart failure or percutaneous coronary intervention.11,34 Most existing case studies were conducted in pediatric and young adult CHD patients. Cooper et al.16 reviewed 14 children and young adults with CHD who underwent 15 lead extraction procedures to remove 21 leads. In 7 of the patients, the CHD had been corrected; 2 of these patients developed complications (29%), including a need for blood transfusion for muscular bleeding and early atrial lead dislodgement. The other 7 patients reported in the study had structurally normal hearts but had cardiomyopathy or primary electrical disease. None of the patients died. Another study of 57 CHD patients with a mean age of around 18 years observed 3 minor bleedings, 3 hematomas, 1 pericardial effusion, 1 surgical extraction, and 10 other minor complications after lead extraction,15 indicating a high complication rate of TLR in this population. A total of 47% of the CHD patients in the study had congenital heart block but the specific CHD types of the remaining patients were not reported. No deaths occurred in any of the 57 patients. The largest sample size in the literature is 144 pediatric CHD patients, reported by Cecchin et al.17 The authors concluded that, although most leads implanted in pediatric CHD patients can be successfully extracted, the procedure carries a risk of severe complications. Several other studies included pediatric and young adult CHD patients undergoing lead extraction procedures.35,36 However, the results were compromised by the small sample size or lack of detailed information on the CHD.
It is difficult to compare the complication rates in this study with those of other studies. Only 2 case studies reported outcomes of TLR in adult CHD patients with a mean age older than 35 years, which is relatively close to the mean age in our study population. In addition, the percentage varies due to differences in complication definition. Khairy et al.13 compared TLR efficacy and complications between 16 adult patients with CHD and 159 patients without CHD. Although the authors concluded that laser lead extraction can be performed with a favorable safety and efficacy profile in selected adult CHD patients, they detected a higher major complication rate in the CHD group than in the non-CHD group (6.3% vs 3%). In a case-control study, the TLR experience was compared between 22 CHD patients and 22 age- and sex-matched non-CHD patients.14 A lower successful extraction rate was reported in the adult CHD patients (74% vs 92%) and only 1 complication occurred in the entire cohort. Again, these studies were limited by their small sample size. In our study, we found a much higher rate of any complication in the CHD group than in the non-CHD group (16.6% vs 10.1%). Hemorrhage and vascular injury were the 2 most common complications, followed by pericardial complications, which were all reported in previous case studies.15,16 Several large population studies revealed a higher complication rate for other cardiac procedures in adult CHD patients, such as implantable cardioverter-defibrillator implantation, indicating that this population is at higher risk of complications.32,37 Unexpectedly, we found that the TLR complication rate in this population was even higher in the 2009 to 2014 period than from 2005 to 2009.
Possible explanations for the increased risk of complications in CHD patients include abnormal venous anatomy, abnormal heart morphology, intracardiac shunting, and abnormal hemodynamics. These conditions are common in complex CHD patients. However, we observed a high complication rate in simple CHD patients, who had 1.5-fold risk for any complication of TLR. The increased rates of complications in simple CHD were mainly due to higher rates of pericardial complications and heart and pericardium repair. One possible explanation is calcium adhesion. Calcification of prosthetic material, often extending into contiguous native tissue such as the myocardium or pericardium, is commonly reported after the surgical repair of CHD.38,39 Calcified adhesions were considered an important factor associated with TLR failure in many previous studies.13,14,40 Given the common use of prosthetic material in simple CHD, such as for patch or valve repair, it is plausible to assume that there is higher incidence of heart tearing due to calcified adhesions.
Although we observed higher complication rates in CHD patients undergoing TLR, these patients were not at increased risk of acute mortality. This is consistent with the various abovementioned case series. Notably, many important factors were not available in the dataset, such as lead age, extraction indication, and types of leads and extraction tools used. Although propensity score matching was performed to balance possible confounders, we could not control for some potential variables. Therefore, the risk estimation results should be cautiously interpreted and there is a need for further large studies with comprehensive variables.
LimitationsThis study has several limitations. First, by using the NIS, a large administrative database, this study presented a cross-sectional assessment of the TLR procedure. Longitudinal data may permit a more comprehensive understanding of the clinical outcomes of CHD patients undergoing TLR. Second, ICD-9 codes exhibit imperfect sensitivity and specificity.41 However, we would expect the bias to be the same for patients with and without TLR complications (nondifferential misclassification), which would tend to bias the measures of association toward the null. Third, although we captured more than 27 000 TLR procedure records, there were few deaths in the CHD group, even with the combination of all types of CHD. We cannot rule out a moderate-sized effect of CHD on in-hospital mortality after TLR. In addition, the exclusion of patients undergoing other invasive procedures, while consistent with the literature,42 may limit the generalizability of the study but focus the analysis on TLR-related complications and minimize confounding. Finally, as mentioned above, complete clinical data were not available, such as procedural technique, lead type and number, lead age, and medication use. Thus, we could not account for confounders.
CONCLUSIONSIn this large population-based analysis, we found that TLR in CHD patients remains a challenge for electrophysiologists. There was a slight increase in TLR procedures conducted in adult CHD patients from 2005 to 2014. Hemorrhage, vascular injury, and pericardial complications were the 3 most common TLR-related complications. Although CHD patients were more likely to be treated in high-volume teaching hospitals, they showed a higher risk of TLR-related complications, but not in-hospital mortality, indicating a greater need for intensive prevention efforts in this high-risk population. These data warrant further investigation of novel and safer approaches for TLR in CHD patients.
CONFLICTS OF INTERESTNone.
- -
Pacemakers and implantable cardioverter-defibrillators are increasingly required in the adult congenital heart disease population.
- -
As more cardiac devices are implanted and more lead failures occur, transvenous lead removal is inevitable in congenital heart disease patients.
- -
Only several small-sized studies have investigated transvenous lead removal in congenital heart disease, with inconsistent results.
- -
In the United States, transvenous lead removal procedures were more commonly performed in simple congenital heart disease patients (∼50%) than in those with complex and unclassified types.
- -
The number of TLR procedures conducted in adult congenital heart disease patients increased slightly from 2005 to 2014.
- -
Hemorrhage, vascular injury, and pericardial complications were the 3 most common TLR-related complications.
- -
Simple and complex congenital heart disease demonstrated around 1.5- and 2.1-fold higher risks of transvenous lead removal-associated complications, respectively.
- -
The in-hospital mortality after transvenous lead removal was similar for patients with and without congenital heart disease.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.08.013