Controversy persists regarding the role of sequential atrioventricular pacing in patients with obstructive hypertrophic cardiomyopathy and disabling symptoms. The aim of this study was to evaluate the effect of pacing on symptoms, dynamic gradient, and left ventricular function in patients with hypertrophic cardiomyopathy.
MethodsFrom 1991 to 2009, dual-chamber pacemakers were implanted in 82 patients with obstructive hypertrophic cardiomyopathy and disabling symptoms despite optimal medical therapy. Sequential pacing was performed with a short atrioventricular delay. Clinical and echocardiographic parameters were measured before and immediately after implantation and after a long follow-up (median, 8.5 years [range, 1-18 years]).
ResultsThe New York Heart Association functional class was immediately reduced after pacemaker implantation in 95% of patients (P < .0001), and this improvement was maintained until the final follow-up in 89% (P = .016). The gradient was significantly reduced after implantation (94.5 ± 36.5 vs 46.4 ± 26.7mmHg; P < .0001) and at final follow-up (94.5 ± 36.5 vs 35.9 ± 24.0mmHg; P < .0001). Mitral regurgitation permanently improved in 52% of the patients (P < .0001). There were no differences in ventricular thickness or diameters, ejection fraction, or diastolic function.
ConclusionsSequential pacing in selected patients with obstructive hypertrophic cardiomyopathy improves functional class and reduces dynamic gradient and mitral regurgitation immediately after pacemaker implantation and at final follow-up. Prolonged ventricular pacing has no negative effects on systolic or diastolic function in these patients.
Keywords
Left ventricular outflow tract (LVOT) obstruction is a key pathophysiological element in obstructive hypertrophic cardiomyopathy (HCM). Between 70% and 75% of patients with symptomatic obstructive HCM have some degree of obstruction, whether at rest or after provocative maneuvers.1 The obstruction can cause symptoms, sometimes disabling, such as exertional dyspnea, angina, and syncope, due to an acute reduction in cardiac output, increased left ventricular (LV) filling pressure, or myocardial ischemia. Patients with LVOT obstruction also have a higher rate of overall mortality and greater risk of sudden cardiac death.2,3 Accordingly, a reduced obstructive gradient is one of the therapeutic targets in obstructive HCM.
Medical therapy of obstructive HCM is based on beta-blockers and verapamil.4,5 These treatments improve symptoms in most patients. In nonresponders to monotherapy with first-line drugs, disopyramide can be useful. Nonetheless, a considerable number of patients remain symptomatic despite optimal medical therapy. Surgical myectomy and alcohol septal ablation effectively reduce the LVOT obstructive gradient and improve symptoms.4 However, these procedures are associated with complications and mortality, especially in older patients or patients with comorbidity. Both techniques also require highly specialized surgeons who are unavailable in most centers.3,6 The symptoms of these patients have also been improved by sequential atrioventricular pacing (SAVP).3,6–11 Preexcitation of the right ventricular apex alters the septal activation sequence, reducing the LVOT gradient and mitral regurgitation severity and possibly attenuating long-term LV remodeling.3,7,11 This therapy has shown a small benefit in randomized clinical trials, particularly in patients older than 65 years.8,9 However, its true long-term efficacy remains a matter for debate due to the possible placebo effect induced by pacemaker implantation.8
The diastolic function of patients with obstructive HCM may show long-term effects from continued DDD pacing with a short atrioventricular delay.12,13 Additionally, prolonged ventricular pacing can negatively impact LV systolic function, leading to long-term clinical decline.13
Currently, and based on controversial data, SAVP has been relegated to a second-line treatment of obstructive HCM.4,5 Its indication is restricted to patients with considerable comorbidity and an unacceptable risk for septal reduction procedures or another indication for dual-chamber pacing.14
Here, we report our 18-year experience in the treatment of patients with obstructive HCM with SAVP, analyzing the effects of this therapy on the LVOT obstructive gradient, mitral regurgitation, functional class, LV remodeling, and systolic and diastolic function.
METHODSPatientsThe present study included 82 patients with obstructive HCM, sinus rhythm, and disabling symptoms despite optimal medical therapy who were treated with SAVP in Hospital Universitario 12 de Octubre between 1991 and 2009. Each patient's treatment was discussed in a multidisciplinary clinical conference before the intervention, and the final decision considered comorbidities, alternative treatment availability, and patients’ wishes. Obstructive HCM was diagnosed using 2-dimensional echocardiographic visualization of unexplained ventricular hypertrophy > 15mm in any myocardial segment. All patients had severe LVOT obstruction (> 50mmHg) on continuous wave Doppler imaging. At the time of patient inclusion, disopyramide was not available in our center.
Study ProtocolInformed consent was obtained from all patients before pacemaker implantation. Clinical and echocardiographic parameters were measured before and immediately after implantation and after a long follow-up (median, 8.5 years [range, 1-18] years). Final data collection was retrospective.
Functional class and angina were evaluated according to the classifications of the New York Heart Association (NYHA) and the Canadian Society of Cardiology, respectively. Information was also collected on history of syncope, presyncope, and heart failure. The echocardiographic variables measured were peak subaortic velocity, peak and mean LVOT gradients at rest and after Valsalva maneuvers, systolic anterior movement of the mitral valve (scored from 0 to 4),11 maximum wall thickness, maximum left atrial diameter in the apical 4-chamber plane, LV ejection fraction (LVEF; measured using the biplane Simpson method), and end-diastolic and end-systolic LV volumes and diameters. Mitral regurgitation was semiquantitatively evaluated based on visual estimation and information from pulsed, continuous, and color Doppler imaging. Regurgitation was classified into 5 grades (0, absent; I, trivial; II, mild; III, moderate, and IV, severe). Diastolic function was assessed using Doppler echocardiography. Also analyzed were the peak velocity of the E and A waves, E/A and E/E’ ratios, pressure half-time, mitral deceleration time of early filling, isovolumic relaxation time, and pulmonary systolic pressure. Calculation of the E/A ratio was omitted in patients with atrial fibrillation during follow-up.
A permanent dual-chamber pacemaker in DDD mode was implanted according to the standard method in all patients, with the ventricular lead placed in the right ventricular apex.3 The atrioventricular delay was determined using echocardiographic evaluation of acute changes in the LVOT and the transmitral filling pattern during pacing (Figure 1). We chose the atrioventricular delay achieving the highest LVOT gradient reduction without excessive shortening of the ventricular filling time, as indicated by the minimal deterioration in the qualitative morphology of the mitral filling pattern on echocardiography. Attempts were made to ensure a ventricular capture greater than 95%, associating this programming with increased treatment with the maximum doses of atrioventricular nodal blocking agents.
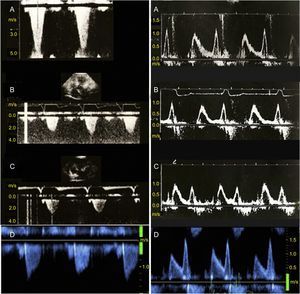
Obstructive gradient of the left ventricular outflow tract (left panels) and transmitral Doppler flow imaging (right panels) in a patient with obstructive hypertrophic cardiomyopathy treated with sequential atrioventricular pacing and followed up for 18 years. A: baseline; B: 6-month follow-up; C: 12-month follow-up; D: final follow-up. Follow-up imaging revealed a permanent reduction in the obstructive gradient of the left ventricular outflow tract (left) and no progression in the baseline diastolic dysfunction (right, A).
Data were collected in regularly scheduled (typically annually) clinical follow-up evaluations that included physical examination, assessment of functional class via clinical history, 12-lead electrocardiogram, and echocardiography. Pacemaker interrogation was performed annually in all patients and 24-Holter monitoring was performed every 1 to 3 years in asymptomatic patients and immediately after new symptom development to evaluate ventricular capture and potential pacemaker dysfunction or arrhythmia. Also recorded were deaths (both cardiac and noncardiac) occurring during follow-up and implant-related complications.
Statistical AnalysisThe normality of the quantitative variables was determined using the Kolmogorov-Smirnov test. Quantitative variables following a normal distribution are expressed as mean ± standard deviation; nonnormally distributed quantitative variables are expressed as median (range). Qualitative variables are expressed as percentages. For comparisons between 2 quantitative variables, the Student t test was used for independent variables if they followed a normal distribution; if not, the Wilcoxon test was used. Qualitative variables were compared using the chi-square test and McNemar's test. Differences were considered statistically significant at P < .05. All statistical analyses were performed using SPSS software (version 17.0, SPSS Inc.).
RESULTSA total of 82 patients (62% women) were evaluated, with a mean age at implantation of 66 years (20-88 years); 82% were older than 65 years. The predominant baseline symptoms were dyspnea (83%), angina (17%), syncope/presyncope (22%), and palpitations (2%). Most patients (93%) were in NYHA III-IV.
The peak gradient was 94.5 ± 36.5mmHg. The mitral regurgitation was moderate or severe in 73% of patients. The mean LVEF was 73% ± 11%, the maximum wall thickness was 22 ± 5mm, and the E/A ratio was 1.02 ± 0.6.
The medical treatments received at implantation were beta-blockers in 76%, calcium antagonists in 38%, and both drugs in 13%.
Twelve patients (15%) had an additional pacemaker indication and 4 (5%) received an automatic defibrillator to prevent sudden cardiac death based on the recommendations in effect at the time of implantation (Table 1).
Baseline Characteristics
| Women, % | 51 (62) |
| Age, y | 66 (22-88) |
| Older than 65 y, % | 67 (82) |
| Advanced functional class (NYHA III-IV), % | 76 (93) |
| Angina, % | 14 (17) |
| Syncope, % | 18 (22) |
| Hypertension, % | 30 (36) |
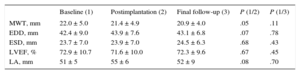
| MWT, mm | 22 ± 5 |
| Peak gradient of LVOT, mmHg | 94.5 ± 36.5 |
| LVEF, % | 72.9 ± 10.7 |
| E/A | 1,02 ± 0.6 |
| Beta-blockers, % | 62 (76) |
| Calcium antagonists, % | 31 (38) |
| Other pacing indication, % | 12 (15) |
| ICD indication, % | 4 (5) |
| Moderate-severe MR, % | 60 (73) |
ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; MWT, maximum wall thickness; NYHA, New York Heart Association functional class.
The mean programmed atrioventricular delay was 120.1 ± 16.2 milliseconds. Complete ventricular capture was confirmed via surface electrocardiogram in all patients.
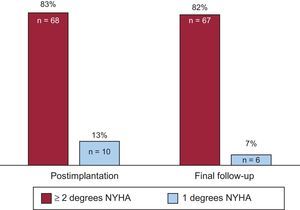
After pacemaker implantation, the functional class improved in 78 patients (95%) (P < .0001): the NYHA functional class dropped at least 2 classes in 68 patients (83%) (from NYHA IV to II in 53% [36 patients] and from NYHA III to I in 47% [32 patients]) and 1 class in 10 patients (from NYHA IV to III in 40% [4 patients], from NYHA III to II in 40% [4 patients], and from NYHA II to I in 20% [2 patients]). At the end of follow-up, 73 patients with a functional class improvement (89% of the total) maintained some degree of improvement: 67 (82%) in at least 2 classes and 6 (7%) in 1 class (P = .016) (Figure 2). No differences were detected in these results according to sex.
A significantly reduced LVOT gradient was seen immediately after pacemaker implantation (94.5 ± 36.5 vs 46.4 ± 26.7mmHg; P < .0001) and at final follow-up (94.5 ± 35.9 vs 36.5 ± 24mmHg; P < .0001) (Figures 3 and 4).
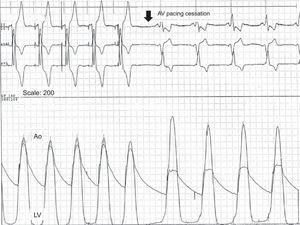
Simultaneous recording of left ventricular and aortic pressures in a patient with left ventricular outflow tract obstruction during sequential atrioventricular pacing and after pacing cessation. During atrioventricular pacing, the dynamic gradient disappears, the peak left ventricular pressure decreases, and the central aorta pressure increases, which improves cardiac output. Ao, aorta; AV, atrioventricular; LV, left ventricle.
After the implantation, the mitral regurgitation severity decreased in 52% of patients, and this improvement was maintained at the end of follow-up (P < .0001). The mean improvement in the mitral regurgitation severity in these patients was 1.4 ± 0.6 severity grades.
An initial nonsignificant tendency for a reduced maximum wall thickness (22 ± 5 vs 21.5mm; P = .05) was not confirmed at the end of follow-up.
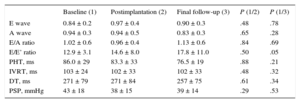
No significant differences were found in the end-systolic and end-diastolic LV diameters or in the LVEF. There were no significant differences in the E and A wave velocities, E/A ratio, pressure half-time, isovolumic relaxation time, and mitral deceleration time of early filling. Additionally, no differences were found in the pulmonary systolic pressure or the left atrial diameter. A nonsignificant tendency for an increased E/E’ ratio was found during follow-up (Tables 2 and 3).
Echocardiographic Parameters 1
| Baseline (1) | Postimplantation (2) | Final follow-up (3) | P (1/2) | P (1/3) | |
|---|---|---|---|---|---|
| MWT, mm | 22.0 ± 5.0 | 21.4 ± 4.9 | 20.9 ± 4.0 | .05 | .11 |
| EDD, mm | 42.4 ± 9.0 | 43.9 ± 7.6 | 43.1 ± 6.8 | .07 | .78 |
| ESD, mm | 23.7 ± 7.0 | 23.9 ± 7.0 | 24.5 ± 6.3 | .68 | .43 |
| LVEF, % | 72.9 ± 10.7 | 71.6 ± 10.0 | 72.3 ± 9.6 | .67 | .45 |
| LA, mm | 51 ± 5 | 55 ± 6 | 52 ± 9 | .08 | .70 |
EDD, left ventricular end-diastolic diameter; ESD, left ventricular end-systolic diameter; LA, left atrium; LVEF, left ventricular ejection fraction; MWT, maximum wall thickness.
Echocardiographic Parameters 2
| Baseline (1) | Postimplantation (2) | Final follow-up (3) | P (1/2) | P (1/3) | |
|---|---|---|---|---|---|
| E wave | 0.84 ± 0.2 | 0.97 ± 0.4 | 0.90 ± 0.3 | .48 | .78 |
| A wave | 0.94 ± 0.3 | 0.94 ± 0.5 | 0.83 ± 0.3 | .65 | .28 |
| E/A ratio | 1.02 ± 0.6 | 0.96 ± 0.4 | 1.13 ± 0.6 | .84 | .69 |
| E/E’ ratio | 12.9 ± 3.1 | 14.6 ± 8.0 | 17.8 ± 11.0 | .50 | .05 |
| PHT, ms | 86.0 ± 29 | 83.3 ± 33 | 76.5 ± 19 | .88 | .21 |
| IVRT, ms | 103 ± 24 | 102 ± 33 | 102 ± 33 | .48 | .32 |
| DT, ms | 271 ± 79 | 271 ± 84 | 257 ± 75 | .61 | .34 |
| PSP, mmHg | 43 ± 18 | 38 ± 15 | 39 ± 14 | .29 | .53 |
DT, deceleration time; IVRT, isovolumic relaxation time; PHT, pressure half-time; PSP, pulmonary systolic pressure.
During follow-up, atrial fibrillation episodes were documented in 28 patients (34%); atrial fibrillation was permanent in 12 of these patients. In these patients, the pacing mode was changed to VVI. Attempts were made to ensure a ventricular capture greater than 95%, associating this programming with increased treatment with the maximum tolerated dose of atrioventricular nodal blocking agents. Greater than 95% ventricular capture was verified by examining the percentage of pacing-sensing activity detected by the device, as well as via 24-hour Holter monitoring to rule out pseudofusions. In 3 of these patients (4%), atrioventricular node ablation was required to ensure correct ventricular capture. There was no significant difference in symptom improvement or in the reduction in the obstructive gradient between patients with atrial fibrillation and those in sinus rhythm and, thus, dual-chamber pacing (P = .7).
During follow-up, 17 patients (21%) died: 4 deaths (all nonresponders to SAVP) were of cardiac origin (3 due to progressive heart failure and 1 due to cardiogenic shock); 13 deaths were attributed to noncardiac causes (cancer in 6 patients, stroke in 1, respiratory problems in 2, neurological disease in 1, and infection in 3); 5 patients (8%) required septal reduction (surgical myectomy) during follow-up due to a lack of response to therapy; 7 patients (8%) had embolic events, probably related to paroxysmal supraventricular arrhythmias.
Only 2 patients (2%) had pacemaker implantation-related complications: 1 generator pocket infection (resolved with antibiotic therapy) and 1 infectious endocarditis requiring device explantation. In the latter patient, after prolonged antibiotic therapy and persistently negative blood cultures, a new device was implanted, with good results.
DISCUSSIONWe present one of the largest published series describing the long-term benefits of SAVP on LVOT gradient reduction and improved symptoms in patients with obstructive HCM.
Follow-up Duration and Patients’ Clinical ProfileDespite the reported benefits of SAVP, its efficacy has been put in doubt by the possible placebo effect of pacemaker implantation.8,9 The reduced obstructive gradient and mitral regurgitation were maintained during the long follow-up, in conjunction with an improved functional class, indicating a direct relationship between the hemodynamic effect and the clinical benefit of the treatment. However, our study design does not allow exclusion of a potential placebo effect. Similar results were obtained in previous studies with long follow-up durations.3,6,15 The symptom reduction may also have been at least partly due to bradycardia improvement in the 12 patients with another pacemaker indication. However, the objective gradient reduction suggests that it was fundamental to symptom improvement.
The few randomized studies evaluating SAVP have shown moderate results. Nonetheless, the clinical benefit is clearer in older patients (> 65 years)8 and those with greater functional limitation.9 Notably, these studies included relatively young patients (mean age, 53 years) and with less severe symptoms (the Pacing in Cardiomyopathy study excluded patients in NYHA IV). The reduced pacing duration in these crossover studies (2-3 months) might be insufficient to permanently reduce the obstructive gradient, as has been indicated.3 Thus, the good results in our population could be explained by the significantly higher mean age of our cohort (66 years; 82% older than 65 years), the higher proportion of patients with disabling symptoms (predominantly NYHA III-IV or limiting angina), and the long pacing duration.
No significant differences were observed in gradient reduction or symptom reduction between patients with permanent atrial fibrillation and those in sinus rhythm during follow-up. The change induced in the ventricular activation pattern during right ventricular apex pacing delays septal contraction and induces premature activation of the papillary muscles and the subvalvular mitral apparatus, reducing the systolic anterior movement of the mitral valve.16,17 This mechanism could explain the benefit of the therapy in patients with atrial fibrillation.
Effect of Sequential Atrioventricular Pacing on Ventricular Systolic and Diastolic FunctionIn our series, there was no significant reduction in the systolic function or increase in the ventricular diameters at the end of follow-up. Diastolic function can also be decreased by SAVP, particularly when there is no previous diastolic dysfunction. In a retrospective study with a 12-month follow-up, patients with diastolic dysfunction (mainly elderly) seemed to benefit more from pacemaker implantation than patients with normal diastolic function.12 There was no significant worsening in diastolic function, probably due to the advanced age of the cohort and the presence of baseline diastolic dysfunction in most patients. On the other hand, no improvement was detected in the baseline diastolic function, as reported previously with a shorter follow-up.3
Effect of Sequential Atrioventricular Pacing on Ventricular RemodelingCardiac pacing can reduce LV thickness.10 Left ventricular remodeling after prolonged pacing in patients with obstructive HCM could explain the late positive response to this therapy.5 No significant reductions were found in the maximum wall thickness, although a tendency for a decrease was seen.
Comparison With Septal Reduction TechniquesIn patients with obstructive HCM whose symptoms are refractory to medical treatment, surgical myectomy is the recommended intervention of choice. Because the results of septal ablation are similar to those of surgical myectomy, ablation is considered an alternative in patients who are not surgical candidates. The third option is considered to be SAVP, mainly in patients with another pacemaker implantation indication (recommendation IIa) or at high risk for septal reduction techniques or when these are unavailable (recommendation IIb).4,5
Notably, SAVP is the most studied invasive strategy in patients with obstructive HCM and has been evaluated in various controlled clinical studies. Although septal reduction techniques have been endorsed in some studies, they may not have been evaluated as exhaustively18 and their indications are based on expert consensus. Additionally, the superiority of surgical myectomy over SAVP has only been shown in highly specialized centers.19 Septal reduction techniques have a high success rate with low associated morbidity and mortality when performed in selected patients and in specialized centers.18 However, the morbidity and mortality associated with surgical myectomy is 15%,20 whereas that of septal ablation is 20% to 40%6,18,20,21 (particularly complete atrioventricular block), markedly higher than that associated with pacemaker implantation. Moreover, up to 20% of patients who undergo septal ablation require further surgery. Ventricular arrhythmias associated with the septal infarct scar have also been described after septal ablation.
Moreover, few centers have the experience and volume necessary to ensure the effectiveness and safety of these techniques, a required condition for their recommendation in clinical practice guidelines. Thus, their availability is limited, in contrast to the wide availability of centers implanting cardiac pacing devices. The economic impact of these techniques, due to the intervention, the hospital stay, and the management of potential complications, could be higher than that associated with pacemaker therapy.
Role of Sequential Atrioventricular Pacing in Current Clinical PracticeLong-term SAVP causes prolonged symptomatic improvements without ventricular function deterioration and facilitates optimization of the medical treatment. For symptomatic patients despite pacing, beta-blocker and calcium antagonist doses can be increased without risk of extreme bradycardia; for therapy responders, the doses of these drugs can be reduced to avoid poorly tolerated adverse effects, such as hypotension.
Regarding the advantages of SAVP in the management of obstructive HCM, Galve et al,6 by applying a therapeutic strategy beginning with SAVP, indicated that only 18% of the patients required a more invasive procedure (septal ablation and/or surgical myectomy). In our series, only 6% of the patients who failed to improve with pacing required septal reduction techniques. Even under these circumstances, a pacemaker could be useful given the relatively common incidence of atrioventricular block associated with these techniques.
LimitationsAlthough the initial study was prospectively planned and the baseline and postimplantation clinical and echocardiographic parameters were collected immediately after implantation, the final data collection was retrospective and could have been affected by the limitations inherent to this type of study.
Functional class was not objectively evaluated. Additionally, symptom evaluation was based on clinical interviews and, primarily, on a subjective NYHA classification. However, this classification is commonly used in clinical practice to guide changes in treatment strategies.
The symptom reduction may have been at least partly due to bradycardia improvement in the 12 patients with another pacemaker indication. However, the objective gradient reduction suggests that it was fundamental to symptom improvement. Similarly, it is possible that the increased doses of beta-blockers/calcium antagonists, which was only feasible after pacemaker implantation and was performed in some patients to ensure adequate ventricular capture, could have helped to improve the gradient and the symptoms.
The findings were not compared with those of a control group. Nonetheless, we believe that the results provide important information for future randomized studies.
To confirm these findings, further studies with larger sample sizes are required, as well as objective measurements of the functional class and longer follow-up periods.
CONCLUSIONSProlonged SAVP in selected patients with obstructive HCM and disabling symptoms could improve functional class and reduce LVOT obstructive gradient and mitral regurgitation both acutely after device implantation and during long-term follow-up, without negative effects on LV systolic and diastolic function.
CONFLICTS OF INTERESTNone declared.