To analyze sex-based differences in clinical characteristics, management, and 28-day and 7-year prognosis after a first myocardial infarction.
MethodsBetween 2001 and 2003, 2042 first myocardial infarction patients were consecutively registered in 6 Spanish hospitals. Clinical characteristics, management, and 28-day case-fatality were prospectively recorded. Seven-year vital status was also ascertained by data linkage with the National Mortality Index.
ResultsThe registry included 449 women and 1593 men with a first myocardial infarction. Compared with men, women were older, had a higher prevalence of hypertension and diabetes, and were more likely to receive angiotensin-converting enzyme (ACE) inhibitors but were less likely to receive beta-blockers or thrombolysis. No differences were observed in use of invasive procedures. More women had non-ST-segment elevation and unclassified myocardial infarction than men (37.9% vs 31.3% and 9.8% vs 6.1%, respectively; both P<.001). Case-fatality at 28 days was similar in women and men (5.57% vs 4.46%; P=.39). After multivariate adjustment, the odds ratio of 28-day mortality for men was 1.06 (95% confidence interval: 0.49-2.27; P=.883) compared with women. After multivariate adjustment, men had higher 7-year mortality than women, hazard ratio 1.93 (95% confidence interval: 1.46-2.56; P<.001).
ConclusionsThere are demographic and clinical differences between men and women with a first myocardial infarction. The short-term prognosis of a first myocardial infarction in this century is similar in both sexes. However, the long-term vital prognosis after a first myocardial infarction is worse in men than in women. These results are observed in both ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction events.
Keywords
During the last 3 decades a large number of studies have focused on the differences between men and women in ischemic heart disease characteristics.1,2 One of the main areas of research has been the differences between the sexes in ischemic heart disease prognosis. Women have a worse short-term prognosis after an acute myocardial infarction (AMI) in most of these studies 3–12 but not in all.13 This poorer short-term prognosis has been mainly observed in ST-segment elevation myocardial infarction (STEMI) cases, particularly in younger women, and seems to be attenuated with age.14 In other studies, the worse prognosis of AMI in women was mainly related to higher age and comorbidity and to differences between the sexes in the pathophysiology15–17 and clinical presentation of the disease,15,18–20 as well as to a lower use of effective drugs and procedures during the acute phase of the disease in women.15,21–26 However, this mortality gap has been decreasing.27
A few studies have analyzed differences between sexes in long-term prognosis after AMI, with conflicting results. Compared with men, women have been reported to have better,28,29 worse,30,31 and similar long-term prognosis.13,32–35
We reported that in the 1990s women had more lethal first AMI than men regardless of age and comorbidity.12 The present study had 3 aims: to determine whether this difference persisted in the first decade of the 21st century, whether there are differences between men and women in 1-year and 7-year mortality after a first AMI and, if so, whether these differences are present in both STEMI and non-ST-segment elevation myocardial infarction (NSTEMI)
METHODSStudy DesignThis is a prospective register of patients with a first AMI undertaken in 6 public hospitals in Spain, with a long-term follow-up of vital status. All patients over 18 years of age who were admitted with a first AMI within 72h of symptom onset from September 2001 to June 2003 were prospectively and consecutively included.
The study was approved by the local ethics committee and all participants were informed and provided signed consent.
Study PopulationThe diagnosis followed the European Society of Cardiology/American College of Cardiology (ESC/ACC) definition36 that AMI is a myocardial necrosis secondary to ischemia. Myocardial necrosis is defined as elevated levels (according to the normal levels as defined in each center) of troponin T or I, or of the creatine phosphokinase-MB fraction, in the presence of symptoms related to myocardial ischemia. Exclusion criteria were a history of previous AMI, residence outside the center's catchment area, and serious illness unrelated to the admission episode that limited the patient's life expectancy.
Variables of InterestA standardized questionnaire administered by trained personnel was used to prospectively gather demographic variables and comorbidities. History of classical risk factors was based on previous diagnosis or treatment or de novo diagnosis during hospital stay using the following criteria: hypertension (systolic blood pressure greater than or equal to 140 or diastolic blood pressure greater than or equal to 90mmHg), diabetes (2 fasting glucose determinations greater than or equal to 126mg/dL or 1 glucose determination greater than or equal to 200mg/dL), dyslipidemia (low-density lipoprotein cholesterol greater than 160mg/dL or high-density lipoprotein cholesterol lower than 35 in men, or 45mg/dL in women). Information related to previous smoking and angina was also recorded by self-report.
Clinical characteristics of the event were recorded, including the delay between symptom onset and hospital monitoring, AMI location, presence of ST-segment elevation on the admission electrocardiogram, appearance of Q waves, and complications such as the development of pulmonary edema or cardiogenic shock or the presence of malignant ventricular arrhythmias within the first 48h (defined as the appearance of ventricular fibrillation or sustained ventricular tachycardia requiring immediate medical attention). Finally, management of the acute event was recorded, including pharmacological treatments during hospital stay and at discharge, reperfusion (thrombolysis or primary percutaneous coronary intervention), and other procedures such as pharmacological or stress-testing techniques to assess the presence of ischemia, echocardiography, coronary angiography to determine the number of vessels with severe lesions, and elective surgical or percutaneous coronary revascularization.
Each participating hospital followed its own protocols for the clinical management of the patients. All of the hospital protocols followed national and international clinical practice guidelines in force at the time of the study.36–38
Events of InterestEvents of interest were defined as 28-day, 1-year, and 7-year mortality, with a follow-up of vital status until December 31, 2009. An individual follow-up was performed in all patients (most of them with a clinical visit and the rest by phone call) to assess 28-day events (angina, reinfarction, stroke, death). Fatal cases were identified through access to the Spanish National Death Registry, an exhaustive and mandatory official database collecting individual data of all deaths in Spain since 1987. This database, made available by the Spanish Health Ministry to public institutions (health care administrations, research centers), provides information on vital status and date of death and can be manually linked with individual patient data in our hospital registries. Patients who did not appear in the Spanish National Death Registry were assumed to be alive at the end of the follow-up.
Statistical AnalysisStudent's t test or the Mann-Whitney U test were used to compare continuous variables and the chi-square test was used to compare categorical variables between 2 groups. Logistic regression and Cox regression were used to determine associations between sex and 28-day or long-term mortality, with adjustment for the confounding variables identified. In the multivariate analyses, different models were built to assess the influence on mortality of different sets of variables, such as age and electrocardiogram pattern (model 1), comorbidities (model 2), the severity of AMI (model 3), management (medical therapy during admission [for 28-day case-fatality] or at discharge [for intermediate and long term mortality]–model 4a–or invasive procedures–model 4b–), and all mentioned variables (model 5). We also tested for the interaction between sex and the electrocardiographic patterns (STEMI and NSTEMI) on 28-day, 1 year, and 7-year prognosis.
In the multivariate analyses, multiple imputation methods39 were performed, using “mi” and “mitools” (R statistical package, version 2.11.1),40 to replace missing values in the adjustment variables and avoid potential selection bias and loss of statistical power. A P-value<.05 was considered as statistically significant.
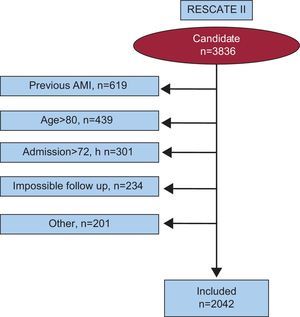
RESULTSThe study included 2042 consecutive first AMI patients, 21.99% of them female (n=449). The flow-chart corresponding to the selection of the patients included in the study is shown in Figure 1.
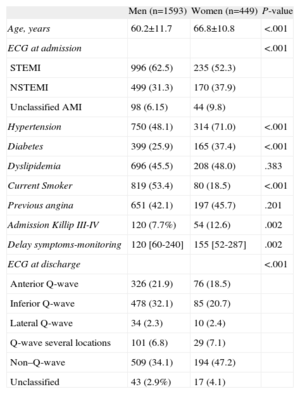
The demographic and clinical characteristics of the patients are presented in Table 1. Women were older, less likely to smoke, and had a higher prevalence of hypertension and diabetes. STEMI was more common in men; the presence of non–Q-wave AMI and Killip III-IV at admission was more frequent in women. Women also showed a longer delay in arriving at the hospital after symptom onset.
Baseline Characteristics of the Patients Included in this Register, by Sex
| Men (n=1593) | Women (n=449) | P-value | |
| Age, years | 60.2±11.7 | 66.8±10.8 | <.001 |
| ECG at admission | <.001 | ||
| STEMI | 996 (62.5) | 235 (52.3) | |
| NSTEMI | 499 (31.3) | 170 (37.9) | |
| Unclassified AMI | 98 (6.15) | 44 (9.8) | |
| Hypertension | 750 (48.1) | 314 (71.0) | <.001 |
| Diabetes | 399 (25.9) | 165 (37.4) | <.001 |
| Dyslipidemia | 696 (45.5) | 208 (48.0) | .383 |
| Current Smoker | 819 (53.4) | 80 (18.5) | <.001 |
| Previous angina | 651 (42.1) | 197 (45.7) | .201 |
| Admission Killip III-IV | 120 (7.7%) | 54 (12.6) | .002 |
| Delay symptoms-monitoring | 120 [60-240] | 155 [52-287] | .002 |
| ECG at discharge | <.001 | ||
| Anterior Q-wave | 326 (21.9) | 76 (18.5) | |
| Inferior Q-wave | 478 (32.1) | 85 (20.7) | |
| Lateral Q-wave | 34 (2.3) | 10 (2.4) | |
| Q-wave several locations | 101 (6.8) | 29 (7.1) | |
| Non–Q-wave | 509 (34.1) | 194 (47.2) | |
| Unclassified | 43 (2.9%) | 17 (4.1) |
AMI, acute myocardial infarction; ECG, electrocardiogram; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
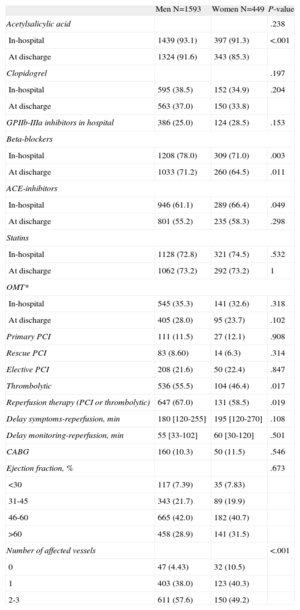
The use of drug therapies and invasive diagnostics or therapeutic procedures by sex is shown in Table 2. The use of beta-blockers and reperfusion (related to a lower use of fibrinolysis) was lower, although the use of angiotensin-converting enzyme inhibitors was higher, in women than in men. No differences between sexes were observed in ejection fraction or the use of invasive procedures, antiplatelets, statins, or optimal pharmacological therapy (defined as a combination of antiplatelets+betablockers+ACE inhibitors+statins). Medical treatments (antiplatelets, statins, ACE inhibitors, or beta-blockers) at discharge were similar to those administered during admission. Men showed a higher proportion of multivessel disease (Table 2).
Pharmacological Therapies In-hospital and at Discharge and Procedures Undertaken During Hospital Admission, by Sex
| Men N=1593 | Women N=449 | P-value | |
| Acetylsalicylic acid | .238 | ||
| In-hospital | 1439 (93.1) | 397 (91.3) | <.001 |
| At discharge | 1324 (91.6) | 343 (85.3) | |
| Clopidogrel | .197 | ||
| In-hospital | 595 (38.5) | 152 (34.9) | .204 |
| At discharge | 563 (37.0) | 150 (33.8) | |
| GPIIb-IIIa inhibitors in hospital | 386 (25.0) | 124 (28.5) | .153 |
| Beta-blockers | |||
| In-hospital | 1208 (78.0) | 309 (71.0) | .003 |
| At discharge | 1033 (71.2) | 260 (64.5) | .011 |
| ACE-inhibitors | |||
| In-hospital | 946 (61.1) | 289 (66.4) | .049 |
| At discharge | 801 (55.2) | 235 (58.3) | .298 |
| Statins | |||
| In-hospital | 1128 (72.8) | 321 (74.5) | .532 |
| At discharge | 1062 (73.2) | 292 (73.2) | 1 |
| OMT* | |||
| In-hospital | 545 (35.3) | 141 (32.6) | .318 |
| At discharge | 405 (28.0) | 95 (23.7) | .102 |
| Primary PCI | 111 (11.5) | 27 (12.1) | .908 |
| Rescue PCI | 83 (8.60) | 14 (6.3) | .314 |
| Elective PCI | 208 (21.6) | 50 (22.4) | .847 |
| Thrombolytic | 536 (55.5) | 104 (46.4) | .017 |
| Reperfusion therapy (PCI or thrombolytic) | 647 (67.0) | 131 (58.5) | .019 |
| Delay symptoms-reperfusion, min | 180 [120-255] | 195 [120-270] | .108 |
| Delay monitoring-reperfusion, min | 55 [33-102] | 60 [30-120] | .501 |
| CABG | 160 (10.3) | 50 (11.5) | .546 |
| Ejection fraction, % | .673 | ||
| <30 | 117 (7.39) | 35 (7.83) | |
| 31-45 | 343 (21.7) | 89 (19.9) | |
| 46-60 | 665 (42.0) | 182 (40.7) | |
| >60 | 458 (28.9) | 141 (31.5) | |
| Number of affected vessels | <.001 | ||
| 0 | 47 (4.43) | 32 (10.5) | |
| 1 | 403 (38.0) | 123 (40.3) | |
| 2-3 | 611 (57.6) | 150 (49.2) | |
ACE, angiotensin-converting enzyme; CABG, coronary artery bypass graft; GPIIb-IIIa, GlicoproteinIIb-IIIa inhibitors; OMT, Optimal Medical Therapy; PCI, percutaneous coronary intervention.
Data are expressed as No. (%) or median [interquartile range].
*Optimal medical therapy in-hospital, combination of acetylsalicylic acid, statins, beta-blockers and angiotensin-converting enzyme inhibitors
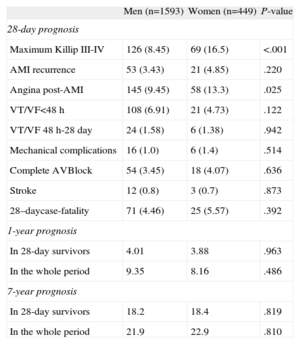
Table 3 shows in-hospital outcome and 28-day case-fatality, stratified by sex. Women showed a higher proportion of pulmonary edema or cardiogenic shock and post-AMI angina than men; 28-day case-fatality was similar in men and women.
Twenty-eight-day Prognosis and 1- and 7-year Mortality Among Patients Included in this Register, by Sex
| Men (n=1593) | Women (n=449) | P-value | |
| 28-day prognosis | |||
| Maximum Killip III-IV | 126 (8.45) | 69 (16.5) | <.001 |
| AMI recurrence | 53 (3.43) | 21 (4.85) | .220 |
| Angina post-AMI | 145 (9.45) | 58 (13.3) | .025 |
| VT/VF<48 h | 108 (6.91) | 21 (4.73) | .122 |
| VT/VF 48 h-28 day | 24 (1.58) | 6 (1.38) | .942 |
| Mechanical complications | 16 (1.0) | 6 (1.4) | .514 |
| Complete AVBlock | 54 (3.45) | 18 (4.07) | .636 |
| Stroke | 12 (0.8) | 3 (0.7) | .873 |
| 28–daycase-fatality | 71 (4.46) | 25 (5.57) | .392 |
| 1-year prognosis | |||
| In 28-day survivors | 4.01 | 3.88 | .963 |
| In the whole period | 9.35 | 8.16 | .486 |
| 7-year prognosis | |||
| In 28-day survivors | 18.2 | 18.4 | .819 |
| In the whole period | 21.9 | 22.9 | .810 |
AMI, acute myocardial infarction; AV, atrioventricular; VF, ventricular fibrillation; VT, ventricular tachicardia.
Data are expressed as No. (%).
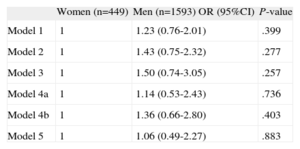
Sex was not significantly associated with 28-day case fatality in the different multivariate models defined (Table 4), and there was no interaction (P=.943) between sex and electrocardiographic patterns (STEMI and NSTEMI) relevant to 28-day case-fatality.
Association Between Sex and 28-day Case Fatality Rate, Adjusted by Various Covariables in Logistic Regression Models
| Women (n=449) | Men (n=1593) OR (95%CI) | P-value | |
| Model 1 | 1 | 1.23 (0.76-2.01) | .399 |
| Model 2 | 1 | 1.43 (0.75-2.32) | .277 |
| Model 3 | 1 | 1.50 (0.74-3.05) | .257 |
| Model 4a | 1 | 1.14 (0.53-2.43) | .736 |
| Model 4b | 1 | 1.36 (0.66-2.80) | .403 |
| Model 5 | 1 | 1.06 (0.49-2.27) | .883 |
95%CI, 95% confidence interval; OR, odds ratio.
Model 1: adjusted for age and electrocardiographic pattern.
Model 2: model 1 plus diabetes, hypertension, smoking, and angina.
Model 3: model 2 plus Killip 3-4, ejection fraction, and number of vessels.
Model 4a: model 3 plus acetylsalicylic acid, clopidogrel, beta-blockers, statins, angiotensin converting enzyme inhibitors, and reperfusion.
Model 4b: model 3 plus elective percutaneous coronary revascularization or coronary artery by-pass.
Model 5: model 3 plus acetylsalicylic acid, clopidogrel, beta-blockers, statins, angiotensin converting enzyme inhibitors, elective percutaneous coronary revascularization or coronary artery by-pass, and reperfusion
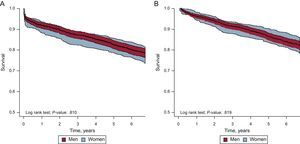
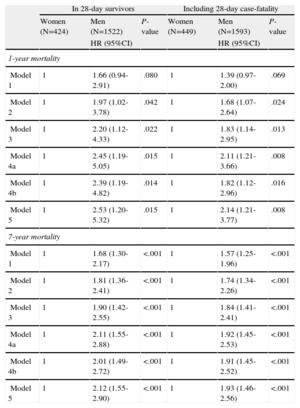
This study achieved 100% follow-up (median, 7.16 years) of patients who survived the acute phase. In the bivariate analysis, there were no sex-related differences in intermediate and long-term mortality (Table 3). The Kaplan-Meier 7-year survival curves for men and women, with their corresponding confidence intervals, are shown in Figure 2.
However, in the multivariate Cox regression models for 7-year mortality, among 28-day survivors a clear association was observed between being male and higher 7-year mortality (Table 5). Depending on the model considered, the probability of death during the 7-year follow-up was 70% higher to more than 200% higher in men than in women. The interaction between sex and the electrocardiographic patterns on 7-year mortality in survivors was not statistically significant (interaction, P=.522).
Association Between Sex and 1-year and 7-year Mortality Rate Adjusted by Various Covariables in Cox Regression Models
| In 28-day survivors | Including 28-day case-fatality | |||||
| Women (N=424) | Men (N=1522) | P-value | Women (N=449) | Men (N=1593) | P-value | |
| HR (95%CI) | HR (95%CI) | |||||
| 1-year mortality | ||||||
| Model 1 | 1 | 1.66 (0.94-2.91) | .080 | 1 | 1.39 (0.97-2.00) | .069 |
| Model 2 | 1 | 1.97 (1.02-3.78) | .042 | 1 | 1.68 (1.07-2.64) | .024 |
| Model 3 | 1 | 2.20 (1.12-4.33) | .022 | 1 | 1.83 (1.14-2.95) | .013 |
| Model 4a | 1 | 2.45 (1.19-5.05) | .015 | 1 | 2.11 (1.21-3.66) | .008 |
| Model 4b | 1 | 2.39 (1.19-4.82) | .014 | 1 | 1.82 (1.12-2.96) | .016 |
| Model 5 | 1 | 2.53 (1.20-5.32) | .015 | 1 | 2.14 (1.21-3.77) | .008 |
| 7-year mortality | ||||||
| Model 1 | 1 | 1.68 (1.30-2.17) | <.001 | 1 | 1.57 (1.25-1.96) | <.001 |
| Model 2 | 1 | 1.81 (1.36-2.41) | <.001 | 1 | 1.74 (1.34-2.26) | <.001 |
| Model 3 | 1 | 1.90 (1.42-2.55) | <.001 | 1 | 1.84 (1.41-2.41) | <.001 |
| Model 4a | 1 | 2.11 (1.55-2.88) | <.001 | 1 | 1.92 (1.45-2.53) | <.001 |
| Model 4b | 1 | 2.01 (1.49-2.72) | <.001 | 1 | 1.91 (1.45-2.52) | <.001 |
| Model 5 | 1 | 2.12 (1.55-2.90) | <.001 | 1 | 1.93 (1.46-2.56) | <.001 |
95%CI, 95% confidence interval; HR, hazard ratio.
Model 1: adjusted for age and electrocardiographic pattern.
Model 2: model 1 plus diabetes, hypertension, smoking, and angina.
Model 3: model 2 plus Killip 3-4, ejection fraction.
Model 4a: model 3 plus acetylsalicylic acid, clopidogrel, beta-blockers, statins, angiotensin-converting enzyme inhibitors, and reperfusion.
Model 4b: model 3 plus elective percutaneous coronary revascularization or coronary artery by-pass.
Model 5: model 3 plus acetylsalicylic acid clopidogrel, beta-blockers, statins, angiotensin-converting enzyme inhibitors, elective percutaneous coronary revascularization or coronary artery bypass, and reperfusion.
We also analyzed 1-year mortality and the results were similar (Tables 3 and 5).
Overall Mortality (Acute, Intermediate and Long-term)The overall 7-year mortality, from symptom onset until the end of follow-up, was similar in men and women (Table 3). However, in the multivariate Cox regression models for 7-year mortality, there was a clear association between male sex and higher overall 7-year mortality (Table 5). The probability of death during the 7-year follow-up was 57% higher to 93% higher in men than in women, depending on the model considered. The results for 1-year mortality were similar (Table 5).
The interaction between sex and the electrocardiographic patterns on overall 7-year and 1-year mortality was not statistically significant.
DISCUSSIONIn this study, the short-term prognosis after a first AMI in the present century was similar in men and women. The women in our registry were older, had greater comorbidity and arrived later at the hospital, although the management of AMI in the acute phase was similar for men and women. Men had a worse long-term prognosis after a first AMI, with a higher 7-year mortality than women. These results were observed in both STEMI and NSTEMI.
Acute Phase PrognosisAlthough direct comparison is not possible because the definition of AMI changed in 2000, a similar study undertaken in 1992-1994 (RESCATE I study) in 4 of the 6 hospitals participating in this study reported that women had a greater short-term risk of death at 28 days and at 6 months after a first AMI than men.12 Moreover, as we showed in a previous study, considering only the patients in RESCATE II with the same diagnostic criteria as RESCATE I, in-hospital mortality decreased by 25% and the 6-month prognosis also improved in the second registry.41
Several factors could contribute to explaining the observed reduction of acute case-fatality, mainly in women, and therefore to the disappearance of the differences between sexes12,41: the mean age of women in the current study was 2 years younger than in the earlier study; although women still arrived later at the hospital than men, the gap between sexes was reduced from 60min to 35min; a lower proportion of women receive reperfusion therapy, but the gap was also reduced, and the proportion of patients treated with thrombolysis or primary percutaneous coronary intervention significantly increased for both men and women, from less than 50% overall (23.9% in women) to almost 66% (58.5% in women); perhaps the most important improvement for women was the one-third decline in pulmonary edema or cardiogenic shock, from 24.8% to 16.5%.
In our study, we observed no differences between the sexes in the management of AMI during hospital stay, unlike other studies.22,23,42 The proportion of patients receiving “optimal pharmacological therapy” (antiplatelets+beta-blockers+ACE inhibitors+statins) and invasive procedures was similar in men and women. However, there were some differences in pharmacological treatments: a lower use of thrombolysis in women, which could be related to the longer delay in arriving at the hospital, and more ACE inhibitors and fewer beta-blockers in women, which could be explained by their higher rate of pulmonary edema and cardiogenic shock.
All these data suggest that the use of evidence-based therapies, including a more invasive approach, in the treatment of AMI in both men and women is associated with a better 28-day prognosis and with the disappearance of the differences between sexes in the acute phase. This similarity in prognosis has been reported in some recent studies13,32 but not in others,31,33 including a recent registry in Chile, which included nearly 50 000 patients. In this registry, younger women have a higher risk of in-hospital mortality due to AMI.44 Moreover, recent Spanish registries have shown higher acute phase mortality rates for women, although men had more readmissions in the long-term follow-up.45 On the other hand, in a large registry in Sweden, the SWEDEHEART study, including more than 46 000 patients admitted with non-ST-segment elevation acute coronary syndrome, outcome with an invasive strategy was similar in men and women, although the follow-up was limited to 1 year.43
Intermediate and Long-term MortalityIn our study, intermediate and long-term (1-year and 7-year) mortality was approximately twice as high in men as in women. Although most studies have not reported mortality differences by sex at 1-year13,32,33,43 or at 3- to 5-year follow-up,30,31,34 higher long-term mortality in men has been reported in other studies with a 3-year28 or a 5-year follow-up.29 Despite the contrasting results, several possible factors could be involved in higher long-term mortality in men. First, there are differences in atherosclerosis progression, especially in the coronary arteries.46 When patients with clinical coronary artery disease are included, women show a lower atheroma burden at the coronary tree than men47 and more often show single-vessel lesion disease; men usually show greater extent of the disease.33 Second, the different coronary atherosclerosis pattern could explain the higher risk of reinfarction in men.33 Finally, we analyzed total mortality. Men have a lower life expectancy than women and causes of mortality other than cardiovascular diseases could also be a factor, especially mortality causes related to smoking, such as pulmonary diseases and certain cancers.
Although the cause of death was not available, we consider that 1-year mortality after an AMI is mainly related to cardiovascular causes, as reported in other studies,48 especially in a young population such as that in our study. We analyzed and compared 1-year and 7-year mortality and observed that the excess of mortality rates in men were similar at 1- and 7-years, suggesting that this excess could be related to cardiovascular causes.
Strengths and LimitationsOur study has several limitations. We have data on in-hospital management and discharge treatment but no information on changes in pharmacological therapy, risk factor exposure or control during the follow-up, or outcomes other than death. Furthermore, although we determined overall 7-year mortality, the specific cause of death was not available. One of the strengths of the study is that the results are based on an exhaustive and consecutive register of hospitalized first AMI patients, with a long follow-up and with detailed clinical information obtained during the hospital stay.
CONCLUSIONSThere are demographic and clinical differences between men and women with a first myocardial AMI. The short-term prognosis of a first AMI in this century is similar in both sexes. However, the long-term vital prognosis after a first AMI is worse in men than in women. These results are observed in both STEMI and NSTEMI.
FUNDINGThis work was partially supported by an unconditional fellowship awarded by Sanofi-Aventis, and by grants from Instituto de Salud Carlos III (Red HERACLES RD06/0009 and CIBERESP).
CONFLICTS OF INTERESTNone declared.