The publication of the EMPEROR-Preserved trial and data on the benefits of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with heart failure (HF) with ejection fraction (EF)> 40% represent a significant step forward in the treatment of HF with preserved EF. Given these results, in February 2022 the US Food and Drug Administration approved the use of empaglifozin in adults with HF with reduced or preserved EF. However, more detailed analysis of the EMPEROR-Preserved trial led to doubts about the effect of empagliflozin in patients with an EF of> 60% this patient group is widely heterogeneous and, probably, a single phenotype cannot be considered in treatment goals or the clinical approach. Moreover, EF occurs on a continuum and classifications of HF according to arbitrary cut-points in EF do not appear consistent with recent evidence, which points to a gradual shift and considerable overlap in underlying mechanisms, phenotypes and treatment response over the spectrum of EF. Enhanced knowledge of pathophysiological mechanisms is essential to establish new therapeutic targets, interpret the results of clinical trials, and develop targeted and effective therapies.
Keywords
An increasing incidence of cardiovascular (CV) risk factors, together with population aging and the longer life expectancy of cardiac patients, has increased the prevalence of heart failure (HF).1 The socioeconomic impact of this condition poses a challenge for our health system. In Spain, HF affects 2.7% of the population older than 45 years and 8.8% of those older than 74 years. In addition, it is the leading cause of hospitalization in the group aged 65 years and older.1 In the United States, HF has an estimated prevalence of 6 million people (≈1.8% of the population).2 It is associated with high mortality (50%-60% at 5 years) and morbidity rates, and a particularly significant reduction in quality of life.3
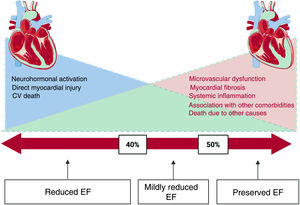
Currently, the most widely used criterion to classify HF patients is left ventricular ejection fraction (EF). This parameter has enabled differentiation between 2 phenotypic patterns of the disease having different clinical characteristics: HF with preserved EF (HFpEF) when the EF is > 50%, and HF with reduced EF (HFrEF) when the EF is <50%. Patients with HFrEF are usually younger, predominantly men, and have a high prevalence of ischemic heart disease. In contrast, the profile of HFpEF patients shows a predominance of women, more advanced age, and the frequent coexistence of CV risk factors, such as hypertension, diabetes mellitus, and atrial fibrillation.3 HFpEF accounts for almost half of all HF admissions worldwide, and the prognosis is as unfavorable as that of patients with reduced EF.
The guidelines of the European Society of Cardiology4 recently established a new HF phenotype, mid-range EF, defined on EF values of 40% to 49%. This patient population shares some characteristics of HFpEF and HFrEF, but in terms of the pathophysiology and especially treatment response, they seem to be closer to those with HFrEF.5 For this reason, the term mildly reduced EF is now preferred over mid-range EF.6 In another group, EF improves and even normalizes with treatment; hence, these individuals are referred to as having recovered EF (HFrecEF).6 It is especially important to maintain optimal medical treatment in these patients, as treatment discontinuation could be related to new EF decline.7
Although the difference seems to be mathematical (> 50% and <50%), recent evidence actually points to a more gradual change and considerable overlap in the underlying mechanisms, phenotypes, and treatment responses across the EF spectrum (figure 1). Up to now, the clinical benefits associated with HF treatment have been limited to patients with HFrEF. HF prognostic drugs have not proven to reduce the combined endpoint of HF hospitalization and CV mortality in HFpEF patients, yielding only modest results, especially in the lower end of preserved EF and in mildly reduced EF (figure 2).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs with hypoglycemic activity initially developed for the treatment of diabetes, are revolutionizing the approach to CV disease. The cardiorenal benefits observed in the main clinical trials led to a clear decrease in undesirable events (table 1). These agents have shown an evident benefit in established CV disease and chronic kidney disease, as well as in HFrEF patients, regardless of whether or not they have diabetes.6 These results have prompted international guidelines to assign SGLT2 inhibitors a class Ia recommendation for treating patients with type 2 diabetes mellitus (DM2) and CV risk, and for patients with DM2 and HFrEF.6
Main clinical trials investigating SGLT2 inhibitors
| Study | MACE | CV death | HF hospitalization | Renal endpoint |
|---|---|---|---|---|
| EMPA-REG OUTCOME, NCT01131676 | 0.86 (0.74-0.99) | 0.62 (0.49-0.77) | 0.65 (0.50-0.85) | 0.54 (0.40-0.75) |
| CANVAS, NCT01032629 | 0.82 (0.72-0.95) | 0.87 (0.72-1.06) | 0.76 (0.52-0.87) | 0.6 (0.47-0.77) |
| DECLARE-TIMI 58, NCT01730534 | 0.9 (0.79-1.02) | 0.98 (0.82-1.17) | 0.73 (0.61-0.88) | 0.53 (0.43-0.66) |
| CREDENCE, NCT02065791 | 0.85 (0.69-1.06) | 0.78 (0.61-1.00) | 0.61 (0.47-0.80) | 0.66 (0.53-0.81) |
| VERTIS CV, NCT01986881 | 0.99 (0.88-1.12) | 0.92 (0.77-1.10) | 0.7 (0.54-0.90) | 0.81 (0.64-0.70) |
| Overall mean | 0.89 (0.84-0.95) | 0.85 (0.78-0.93) | 0.68 (0.61-0.76) | 0.62 (0.56-0.70) |
CV, cardiovascular; HF, heart failure; MACE, major adverse cardiovascular events
The results of recent studies such as EMPEROR-Preserved8 seem to indicate that the clinical benefits of SGLT2 inhibitors also extend to patients with HFpEF (for the first time in this condition). On February 24, 2022, the United States Food and Drug Administration (FDA) approved empagliflozin to reduce the risk of CV death and HF hospitalization, whatever the EF status. This article reviews the available evidence, the various mechanisms of action, and the biological effect of SGLT2 inhibitors in HFpEF patients.
DEFINITION OF HFpEFIn the new guidelines, HFpEF is clinically defined based on the following: a) signs and symptoms of HF; b) EF ≥ 50% in the absence of a history of reduced EF; c) other conditions occurring with preserved EF have been ruled out; and d) evidence of increased ventricular filling pressures determined by invasive techniques or indirectly through the E:e’ ratio, increased atrial volume, or elevated natriuretic peptide values.6 However, the diagnostic criteria for HFpEF may have certain limitations:
- •
HFpEF patients are a heterogeneous group with a diverse pathophysiology. In all probability, the condition cannot be treated as a single phenotype when deciding the clinical approach and therapeutic goals.
- •
The threshold used to categorize EF as being reduced or preserved has changed over time (from 40% up to 50%).
- •
The EF value is highly reliant on cardiac preload and afterload, and its measurement varies considerably depending on the imaging technique used. Although cardiac magnetic resonance imaging is considered the reference standard for this purpose, echocardiography is the most widely used technique because of its accessibility and low cost.6
- •
Several studies using myocardial deformation techniques have convincingly shown that a preserved EF value is not always synonymous with preserved left ventricular systolic function.9
- •
Furthermore, cardiac systolic function is a dynamic factor that can vary over time. The trajectory or changes occurring in the EF may have more weighty implications than a single measurement at a given time point.
- •
Natriuretic peptide values tend to be lower in HFpEF, mainly due to milder diastolic wall stress and the high obesity rate in these patents. There are some indications that adipose tissue metabolizes the brain natriuretic peptide (BNP) molecule; hence, BNP values may be lower in obese patients.10
In HFrEF, several treatments are available to curb overactivation of the neurohormonal system, inherent to this disease. The mainstays of treatment include SGLT2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and neprilysin inhibitors. Currently, the priority is to block all pathways related to HF progression rather than to reach maximum doses of any of these drugs, which would impede prescription of the others.6
The benefits of SGLT2 inhibitors are quickly evident. The DAPA-HF11 clinical trial (4744 patients, 18-month follow-up) showed a reduction in the composite endpoint of CV death or worsening HF with dapagliflozin use, recording a significant improvement after only 28 days of treatment (hazard ratio [HR]=0.51; 95% confidence interval [95%CI], 0.28-0.94). Similarly, the EMPEROR-Reduced study (3730 patients, 16 months) reported a 58% reduction in the relative risk of death, HF hospitalization, or HF emergency visit after 12 days of treatment with empagliflozin.
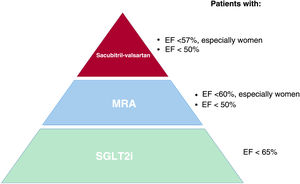
Unlike HFrEF, the therapeutic options for HFpEF are quite limited. The approach in these patients focuses on prevention and control of comorbidities, relief of congestive symptoms, and cardiac rehabilitation. Some benefits have been described with administration of mineralocorticoids or neprilysin inhibitors, but the effects have been modest and only seen in certain patient subgroups (figure 2).
Treatment of HFpEF with spironolactone in the TOPCAT12 study (3445 patients, 3-year follow-up, EF> 45%) did not significantly reduce the primary composite endpoint (time to CV death, cardiac arrest, or HF hospitalization). In a post hoc analysis, however, a 4-fold difference in this endpoint was found in patients in Russia and Georgia compared with patients in the United States, Canada, Brazil, and Argentina. Some authors have noted that the Russian and Georgian patients did not meet all the criteria for HF (if the disease is not present, there will be no improvements with treatment) and have questioned treatment adherence (no improvements if the drug is not taken), as the patients showed no increases in potassium, creatinine, or canrenone, the active metabolite of spironolactone.13
Finally, there was no decrease in the primary endpoint (CV death and HF hospitalization) with sacubitril-valsartan administration in the PARAGON-HF14 study (4822 patients, EF> 45%), although there was a significant improvement in the patients’ functional class and a delay in renal function worsening. In the subgroup analysis, a possible benefit was observed in women (HR,0.73; 95%CI, 0.59-0.90) and in patients with an EF below the median (EF <57%) (HR,0.78; 95%CI, 0.64-0.95) (figure 2).
All in all, none of the treatments investigated to date in HFpEF have yielded an overall reduction in the primary endpoints of HF hospitalization or CV death, which is why publication of the EMPEROR-Preserved study marks a new turning point in this issue (table 2).
Main clinical trials in heart failure with preserved ejection fraction
| Study | Year | EF (%) | Treatment | Control | Primary endpoint | RRR |
|---|---|---|---|---|---|---|
| CHARM-Preserved, NCT00634712 | 2003 | > 40 | Candesartan | Placebo | CV death + HF hospitalization | −11% |
| PEP-CHF15 | 2006 | > 40 | Perindopril | Placebo | Death + HF hospitalization | −8% |
| I-Preserve, NCT00095238 | 2008 | > 45 | Irbesartan | Placebo | Death + HF hospitalization | −5% |
| TOPCAT, NCT00094302 | 2014 | > 45 | Spironolactone | Placebo | CV death + HF hospitalization + cardiac arrest | −11% |
| PARAGON-HF, NCT01920711 | 2019 | > 45 | Sacubitril-valsartan | Valsartan | Death + HF hospitalization | −13% |
| EMPEROR-Preserved, NCT03057951 | 2021 | > 40 | Empagliflozin | Placebo | CV death + HF hospitalization | −21% |
| PRESERVE-HF, NCT03030235 | 2021 | > 45 | Dapagliflozin | Placebo | Quality of life | 6 points KCCQ |
| CHIEF-HF, NCT04252287 | 2021 | > 45 | Canagliflozin | Placebo | Quality of life | 4.3 points KCCQ |
| DELIVER, NCT03619213 | 2022? | > 40 | Dapagliflozin | Placebo | CV death + HF hospitalization | Ongoing |
CV, cardiovascular; EF, ejection fraction; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; RRR, relative risk reduction
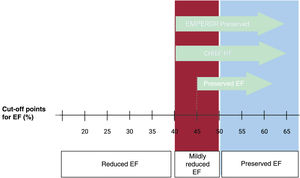
The EMPEROR-Preserved trial examined the effect of empagliflozin administration in HFpEF patients (5988 patients, 26.2 months of follow-up). The cutoff point to define HFpEF was an EF value > 40%, as measured with any imaging technique (figure 3) (including ventriculography and nuclear medicine). Patients had to have an EF determination> 40% within the previous 6 months and no history of EF <40% during a stable period. Therefore, the authors considered the possibility of including not only patients with preserved EF, but also those with mildly reduced EF and, very likely, with recovered EF.
The results showed a 21% decrease vs placebo in the composite endpoint of CV death or hospitalization for HF (HR,0.79; 95%CI, 0.69-0.90; P=.003) based on a number needed to treat of 31 patients. This effect was mainly related to a 29% reduction in the risk of HF hospitalization (HR,0.71; 95%CI, 0.60-0.83). The magnitude of the effect on hospitalization risk with empagliflozin use in HFpEF patients was similar to previous findings in HFrEF in the EMPEROR-Reduced study (29% vs 31%). On subgroup analysis, the effect was significant in both diabetic and nondiabetic patients.
Two main points should be noted regarding this study: first, the clinical benefit was attenuated in patients with EF> 60% (HR,0.87; 95%CI, 0.69-1.10), and second, almost 1 in every 3 patients had an EF value <50%. According to the latest guidelines definition, these patients would not be considered to have HFpEF, but rather HF with mildly reduced EF (figure 3).
In the group with EF 40% to 49% (1983 patients), there was a smaller percentage of women (33%), and a higher percentage of diabetes, ischemic heart disease, and treatment with beta-blockers, angiotensin-converting enzyme inhibitors, and mineralocorticoid receptor antagonists. The decrease in the composite was greater in this subgroup, 29% (HR,0.71; 95%CI, 0.57-0.88), and also derived from first HF hospitalizations. Furthermore, the total of HF hospitalizations (first and successive hospitalizations) was significant (HR,0.57; 95%CI, 0.42-0.79; P <.001).
When HF patients with mildly reduced EF were excluded, the group with definite HFpEF (EF > 50%) consisted of 4005 patients. The clinical features of these patients were slightly different from those described above: 50% were women, and patients were significantly older and had lower NT-proBNP values. The reduction in the primary endpoint continued to be significant, at 17% (HR,0.83; 95%CI, 0.71-0.98; P=.024), and was mainly attributable to first HF hospitalizations (HR,0.78, 95%CI, 0.64-0.95, P=.013). However, unlike the previous group, the total of hospitalizations showed no significant decrease.
After exclusion of HFpEF patients with EF> 60%, in whom the effect of the drug seemed to be attenuated, a significant reduction in the primary endpoint was also seen in patients with EF between 50% and 60% (HR,0.80, 95%CI, 0.64–0.99). In a subsequent analysis, Milton Packer compared the results of the EMPEROR-Preserved study and PARAGON-HF in patients with EF values between 52.5% and 62.5% (interval established to equate the 2 studies).16 Empagliflozin administration led to significant reductions in the composite of CV death and HF hospitalization, in first HF hospitalizations (HR,0.68; 95%CI, 0.51–0.89), and in total hospitalizations (first and recurrent), whereas the risk of CV death alone did not decrease. In contrast, the PARAGON-HF study found no significant reductions in any of the endpoints in this EF range.16
EMPEROR-POOLEDThe EMPEROR-Pooled17 study (9718 patients) determined the effect of empagliflozin over the entire spectrum of EF values. The authors jointly evaluated the 2 largest trials investigating this drug in HF patients: EMPEROR-Reduced (EF <40%) and EMPEROR-Preserved (EF> 40%). The 2 trials had the same primary endpoint (composite of CV death and HF hospitalization) and 2 main secondary endpoints (HF hospitalization and worsening renal function). The statistical approach used in EMPEROR-Pooled had already be designed before enrollment started in either of these 2 studies.
Empagliflozin use resulted in a decrease in first HF hospitalizations and total HF hospitalizations (first and recurrent) to a similar extent (25%-35%) in patients with EF <25% to <65% (figure 2). However, the group with highest EF (> 65%) showed no benefits (HR,1.03; 95%CI, 0.67–1.60).
Regarding the subgroup with EF > 65%, the authors noted that they accounted for less than 10% of the total number of patients and total number of events occurring in the study. Hence, they considered that the associated results might lack precision and once again, mentioned the need for studies specifically focused on this type of patient. Second, most of the patients had specific clinical characteristics: a high prevalence of atrial fibrillation, low levels of natriuretic peptides, and a higher percentage of women, older age, and hypertension. Natriuretic peptides were only slightly elevated relative to the value in the inclusion criteria. The authors hypothesized that congestive symptoms such as dyspnea in this group might have been related less to HF and more to atrial fibrillation, obesity, lung disease, or other comorbidities. The doubts raised regarding the diagnosis could explain the lack of effectiveness of a drug investigated precisely as HF treatment.
DUAL SGLT1 AND SGLT2 INHIBITIONThe SCORED18 and SOLOIST-WHF19 studies assessing sotagliflozin were not specifically designed to determine the effect of this drug in HFpEF patients, but the results obtained are very promising.19 Sotagliflozin is a dual SGLT2 and SGLT1 receptor inhibitor. In contrast to the predominantly renal expression of SGLT2, the SGLT1 receptor is expressed mainly in the intestine and to a lesser extent in the S3 segment of the renal proximal tubule and the heart.20 Inhibition of both these receptors increases the percentage of renal glucose elimination through the proximal tubule and, owing to the SGLT1 receptor, also inhibits intestinal absorption of glucose. Another difference with respect to the SGLT2 receptor is that SGLT1 expression has been demonstrated in the myocardium and is seen to significantly increase during ischemia. Its role at this level is not yet fully understood, but experimental studies indicate that myocardial SGLT1 inhibition may have a potential cardioprotective effect.20
The favorable safety profile and efficacy of sotagliflozin has led to its approval by the European Medicines Agency for the treatment of type 1 diabetes mellitus. However, as in other SGLT2 inhibitors, the metabolic benefits of the drug do not seem to depend exclusively on its activity in controlling blood glucose levels. Sotagliflozin has proven to reduce the risk of HF hospitalizations in diabetic patients with chronic kidney disease (SCORED) and decompensated HF (SOLOIST-WHF). Furthermore, the benefits obtained were significant after only a few weeks of treatment.
One novel aspect of the SOLOIST-WHF study (1222 patients, 9-month follow-up) was administration of a drug of this type in patients with decompensated HF (first dose given even before hospital discharge in 48% of patients). This differentiates it from DAPA-HF or EMPEROR, both focused on patients with stable HF. Sotagliflozin led to a 33% reduction (HR,0.67; 95%CI, 0.52–0.85; P <.001) in the composite primary endpoint of CV mortality, HF hospitalizations, and HF emergency visits, with a 26% decrease in HF hospitalizations (HR,0.64; 95%CI, 0.49-0.83; P <.001). There were no significant reductions in CV deaths or other-cause mortality. Nonetheless, it is important to bear in mind that the study was stopped earlier than planned due to funding problems. This resulted in a significant decrease in the number of patients included and forced a change in the original primary endpoint (CV death and HF hospitalization).
Another aspect to highlight is the low percentage of patients with EF > 50% (≈20%). However, the subgroup analysis showed that sotagliflozin had a significant effect in both HFrEF (EF <50%) (HR,0.72; 95%CI, 0.56-0.94) and HFpEF (EF> 50%) (HR,0.48; 95%CI, 0.27-0.86) patients.
PATIENT-REPORTED BENEFITS IN QUALITY OF LIFEHF has a huge impact on quality of life, leading to similar or even greater limitations than those occurring in chronic obstructive pulmonary disease, pulmonary hypertension, a history of stroke, or dialysis requirement.21 For this reason, in addition to the endpoints of mortality and hospital admissions, patient-perceived improvement in quality of life is becoming an essential element in HF treatment. In some situations, it may be even more valued than the improvement in survival, as in the case of very elderly patients or those with numerous comorbidities. 22
Use of clinical questionnaires to measure the effects of treatment from the patient's perspective, generically termed patient-reported outcomes, is increasingly more common in clinical trials. They consist of a series of questions grouped into areas or domains (eg, physical limitation, social limitation, frequency of symptoms). The most specific questionnaires for HF are the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ). Recently, the FDA cataloged the KCCQ as a primary endpoint (clinical outcome assessment) for clinical trials.22
The PRESERVE-HF23 study, investigating dapagliflozin use in HFpEF (324 patients, 12-week follow-up), demonstrated improvements in the symptoms, physical limitations, and functional capacity related to the disease. The EF cutoff point was > 45% (mean, 60%). The treated group showed a 5.8-point improvement in the KCCQ clinical score compared with those receiving a placebo.
For its part, canagliflozin led to reductions in HF-associated symptoms in the CHIEF-HF24 study (476 patients, 12-week follow-up), regardless of the EF or the presence of diabetes mellitus (DM). Among the total included, 59% of patients had HFpEF (defined as EF > 40% in this study) (figure 3). The total KCCQ test score, established as the primary outcome measure, was significantly higher after only 2 weeks of follow-up, and the results remained significant up to completion of the study (P=.016).
Unsurprisingly, the functional and clinical benefits reported in these studies were accompanied by improvements in the patients’ quality of life. The EMPA-TROPISM25 study (HFrEF patients, mean EF 36%) found that structural and functional changes due to ventricular remodeling were associated with improvements in functional capacity and quality of life, as measured by the KCCQ.26 These results, together with the DEFINE-HF,27 DAPA-HF, and EMPEROR-Reduced findings, further strengthen the concept of a class effect with SGLT2 inhibitors, this time in terms of quality of life.
BIOLOGICAL EFFECT OF SGLT2 INHIBITORS IN HFpEFHFpEF is a complex syndrome with multiple etiologies. It is often associated with various morbidities, such as DM2, obesity, aging, and chronic kidney disease. The pathophysiology of HFpEF is not yet fully understood, precisely because of this complicated interrelationship between comorbidities, and because of the relative scarcity of experimental models that can accurately reproduce an HFpEF model. The main biological processes that characterize this condition are systemic inflammation, increase and dysfunction of epicardial adipose tissue, coronary microcirculation changes, myocardial fibrosis, and vascular stiffness. These factors lead to impaired vascular and ventricular compliance, which (especially when accompanied by impaired renal function) increases cardiac filling pressures and results in dyspnea despite EF values in the preserved EF range. As SGLT2 inhibitors have shown a metabolic effect on several of the mechanisms involved in HFpEF, it is plausible that this drug class could provide clinical benefits not only in HFrEF, but also in HFpEF
When added to previous antidiabetic treatment, the hypoglycemic effect of SGLT2 inhibitors through their glucosuric action is modest (0.5%-1% reduction in glycated hemoglobin [HbA1c]). Nonetheless, in recent years, SGLT2 inhibitors have shown benefits in atherosclerotic disease, HF, total mortality, CV mortality, and progression of chronic kidney disease. Because of the magnitude of these effects and the rapid separation of the event curves in most related clinical trials, it is questionable to attribute the protective effect of these drugs exclusively to improved glycemic control. Several experimental studies have shown that SGLT2 inhibitors have a wide variety of metabolic effects that can reduce (or even reverse)25 the pathophysiological deterioration of HF.
In an HFrEF experimental model, our working group demonstrated an increase in myocardial consumption of ketone bodies related to empagliflozin treatment.28 The higher presence of plasma ketone bodies associated with SGLT2 inhibitors provides the heart with a more efficient energy substrate than glucose or fatty acids.29 Better energy efficiency would be especially useful in situations of energy decline, such as HF or myocardial ischemia, where it could help mitigate inflammation and fibrosis.30 Specifically in HF patients, an increase in ketone bodies has resulted in better ventricular contractility.31 In our experimental model, optimization of metabolic status was the basis for explaining the regression in adverse remodeling, reductions in volumes and hypertrophy, and improvements in both systolic28 and diastolic32 function. These findings were later translated to the clinical setting in the EMPA-TROPISM study25 including nondiabetic HFrEF patients. In addition to the adverse remodeling regression determined by cardiac magnetic resonance, empagliflozin administration was associated with an increase in peak oxygen consumption and improvements in quality of life measures.27
Other potential mechanisms of action (not mutually exclusive) have been proposed, such as reductions in epicardial adipose tissue, interstitial myocardial fibrosis, and aortic stiffness,30 improvements in inflammatory parameters, and optimization of iron metabolism.
EPICARDIAL ADIPOSE TISSUEObesity is a major risk factor for HFpEF,33 as increased fat deposit can be particularly detrimental in certain body regions. This is the case of epicardial and visceral adipose tissues, which act as metabolically active organs with an impact on the heart and vasculature. Epicardial adipose tissue is in direct contact with the myocardium and atria, immediately below the visceral layer of the pericardium. It surrounds the coronary arteries and uses the same microcirculation as the heart muscle, which favors greater interaction. Epicardial fat has a dual function. Under normal conditions, it secretes cytokines with a cardioprotective effect such as adiponectin. However, in certain situations, its metabolic activity changes and it becomes a focus for inflammation, reflecting systemic inflammatory changes and transferring them to the myocardium.34 An increase in epicardial proinflammatory cytokines promotes macrophage migration, endothelial dysfunction, and atherosclerotic plaque formation. Furthermore, it impairs diastolic function through uncoupling of calcium channels and desensitization of beta-adrenergic receptors, and activates fibrosis and remodeling of the extracellular matrix. There is a clear relationship between the thickness of the epicardial fat layer and the degree of myocardial inflammation and fibrosis.34
The epicardial fat volume is significantly greater in HFpEF patients, and many of the comorbidities associated with HFpEF, such as obesity, diabetes, and chronic kidney disease, also show epicardial fat increases.33 In HFrEF, the relationship is less clear, as both high and very low epicardial fat values have been reported in these patients.34 In addition, excess epicardial fat has been associated with a poorer prognosis in HFpEF.33–35 A significant increase in any cause deaths and HF hospitalizations36 has been reported in patients with preserved or mildly reduced EF values, regardless of the body mass index and other comorbidities. Obese patients with a high epicardial fat volume showed a greater relative risk of events than those with a lower volume. In another study in obese HFpEF patients, increased epicardial fat was associated with more marked hemodynamic changes: higher filling pressures, more severe pulmonary hypertension, and poorer functional capacity.35
In the EMPA-TROPISM25 clinical trial, a significant decrease in the total epicardial adipose tissue volume was seen after 6 months of empagliflozin treatment.30 This reduction was accompanied by an improvement in several inflammatory biomarkers, myocardial fibrosis, and aortic stiffness.30 Dapagliflozin, ipragliflozin, luseogliflozin, and canagliflozin, as well as some GLP-1 receptor agonists,34 have also led to reductions in epicardial adipose tissue, similar to the effects reported in EMPA-TROPISM.
The precise mechanism of action by which SGLT2 inhibitors act at this level remains to be defined, but it does not seem to depend entirely on weight loss.30,37 Unlike the myocardium, SGLT2 receptor expression has been found in adipose tissue samples from patients undergoing cardiac surgery.38 In these same clinical samples, dapagliflozin was found to induce higher glucose uptake, lower expression of inflammatory cytokines, and better differentiation of epicardial adipocytes.
DIASTOLIC FUNCTIONDiastolic dysfunction is one of the most common events occurring in the development of HFpEF. In 1998, diastolic HF was actually the first term used to refer to patients with congestive symptoms, EF in the normal range, and no left ventricular dilatation.39 In contrast to patients with HFrEF, most HFpEF patients have normal ventricular volumes and elevated filling pressures at rest or on exertion.
Increasing evidence indicates that SGLT2 inhibitors have a direct effect on diastolic function. In diabetic patients with preserved EF, canagliflozin has proven to reduce the E/e’ ratio, a marker of diastolic dysfunction, after 3 months of treatment.40 In a small study including diabetic patients with established CV disease,41 empagliflozin led to reductions in the ventricular mass and significant improvements in diastolic function on tissue Doppler, also at 3 months.
The benefits of empagliflozin on diastolic function have been consistently investigated in preclinical models. Our working group used an experimental HFrEF model with nondiabetic pigs to study the effect of empagliflozin on diastolic function independently of glycemic control. Animals were randomized to receive empagliflozin or placebo, and were reassessed at 2 months. Diastolic function was evaluated by echocardiography, magnetic resonance imaging, cardiac hemodynamic study, and analysis of myocardium and blood using histological and molecular biology techniques. At completion of follow-up, empagliflozin-treated animals showed significantly better diastolic function than those treated with placebo. The imaging findings correlated with the histology and molecular biology results, all of which reflected a reduction in myocardial fibrosis and oxidative stress.32
IRON METABOLISMAnemia and iron deficiency are common comorbidities in HF, and both conditions are independently associated with the patient's prognosis and clinical status.42 Iron deficiency is found in up to up to 59% of patients, and even in the absence of anemia it leads to a decline in functional capacity and quality of life, and an increased risk of hospitalization and death.43
In HFrEF, intravenous ferric carboxymaltose treatment proved to relieve symptoms, improve quality of life and functional capacity, and reduce hospitalizations. In a substudy of the EMPA-TROPISM trial, our working group also evaluated the effect of empagliflozin treatment on myocardial iron content measured by cardiac magnetic resonance imaging.44 After 6 months of empagliflozin administration, there was a significant reduction in T2* values compared with placebo (-1.25±2.4 vs 0.2±2.6ms; P=.007), indicating recovery of myocardial iron (T2* values and tissue iron content are inversely proportional). In addition, the T2* changes correlated with reductions in ventricular volumes and hypertrophy and with increases in the EF and peak oxygen consumption. Similarly, using cardiac magnetic resonance imaging, the Myocardial-Iron trial demonstrated that intravenous iron administration leads to significant short-term T2* changes, which were also related to improvements in the EF.45
To date, most studies showing iron deficiency in HF and evaluating the effectiveness of various treatments have focused on HFrEF patients. However, the findings in some patient series indicate that the prevalence of iron deficiency may be even higher in the group with HFpEF.46 Some studies investigating intravenous iron administration in patients with preserved EF47,48 have also reported benefits in quality of life and functional capacity. It is a known fact that functional iron deficiency is associated with inflammatory processes, and several inflammatory pathways are actively involved in the pathophysiology of HFpEF. Interleukin (IL) 6 and to a lesser degree IL-1β stimulate hepcidin expression through the JAKSTAT3 transcriptional pathway. Hepcidin inhibits ferroportin 1 by blocking iron uptake in the intestinal mucosa and by mobilizing the cellular deposits. Although improvements in iron metabolism could also be a plausible therapeutic objective in HFpEF, new studies focused specifically on this type of patient are still needed.
CONCLUSIONSTreatment of HF patients has undergone considerable changes in the last 5 years. SGLT2 inhibitors, drugs initially considered to provide antidiabetic treatment, have led to reductions in hospitalizations and mortality in HF patients, whether or not they have diabetes. The metabolic gain of these agents is independent of glycemic control and complementary to other therapeutic lines showing prognostic benefit.
The following are the most important questions raised by SGLT2 inhibitor use in the treatment of patients with HF:
- •
Should all patients be treated regardless of their blood glucose status? The answer is yes. The biological effect of SGLT2 inhibitors does not depend on glycemic control and benefits have been shown in both diabetic and nondiabetic patients.
- •
How and when should SGLT2 inhibitor treatment be started? SGLT2 inhibitors are one of the 4 pillars of HF treatment, together with beta-blockers, mineralocorticoid receptor antagonists, and sacubitril-valsartan. The current priority is to block the 5 pathways related to HF progression rather than attempting to reach maximum doses of any of the drugs, which would prevent prescription of the others. SGLT2 inhibitors have shown a prompt, additional benefit to that of other treatments, which would justify their early, first-line administration for HF, including in newly diagnosed and hospitalized patients.
- •
Should all HF patients be treated regardless of their EF value? Probably, yes. SGLT2 inhibitors may not be effective in all patients with HFpEF, but the EMPEROR-Preserved study has provided a step ahead for understanding the mechanisms of action and diverse phenotypes of this disease. The concerns regarding the higher values in the EF spectrum, also seen in relation to other drugs, underscore the need for clearly targeted studies, explicitly designed for HFpEF. Ideally, these new studies should use stricter criteria for measuring EF, which should be assessed by cardiac magnetic resonance imaging and at a time point much closer to the date of randomization than has been done in previous studies (EMPEROR, 6 months; DEFINE, 12 months). Furthermore, rather than a simple mathematical differentiation based on EF values, new classification criteria based on the etiology or pathophysiology of the disease will likely be of help to better interpret the results of clinical trials and establish more precise therapeutic targets. For the time being, and pending new studies that confirm a possible class effect of SGLT2 inhibitors also in HFpEF, the available findings should be combined with control of comorbidities, promotion of a healthy lifestyle, and cardiac disease prevention and rehabilitation in these patients.
The recently published guidelines of the American College of Cardiology/American Heart Association (April, 2022)49 provide the first support for SGLT2 inhibitor treatment specifically in HFpEF patients, with a class IIa recommendation. Administration of mineralocorticoid receptor antagonists and neprilysin inhibitors is also considered reasonable in this group, although the evidence is weaker (IIb recommendation). As to HF classification, the guidelines highlight the dynamic nature of the EF and the prognostic implications of changes in this measure over time. The definition of new groups has been refined, and there are specific recommendations for patients with mildly reduced EF and with recovered EF as possible transition phenotypes.
These new recommendations are in line with our commentary and they highlight the need for new studies and new classification criteria more in keeping with the pathophysiology of HF. Better characterization of affected patients is essential to develop successful treatments for this condition, particularly in the group with a preserved EF.
FUNDINGJ.A. Requena-Ibáñez received funding from the Spanish Society of Cardiology through a mobility grant (SEC/PRS-MOV-INT 21/004). C.G. Santos-Gallego and J.J. Badimón declare that they have received no funding.
AUTHORS’ CONTRIBUTIONSAll authors have participated in writing the manuscript and have approved the final version.
CONFLICTS OF INTERESTThe authors state that they have no potential conflicts of interest related to this study.