The Spanish Registry of Acute Aortic Syndrome (RESA) was launched in 2005 to identify the characteristics of acute aortic syndrome (AAS) in Spain. The aim of this study was to analyze the differences in management and mortality in the 3 RESA iterations.
MethodsWe analyzed data from patients with AAS prospectively included by 24 to 30 tertiary centers during the 3 iterations of the registry: RESA I (2005-2006), RESA-II (2012-2013), and RESA III (2018-2019).
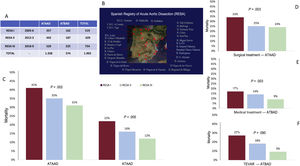
ResultsAAS was diagnosed in 1902 patients (74% men; age, 60.7±12.5 years): 1329 (69.9%) type A and 573 (30.1%) type B. Comparison of the 3 periods revealed that the use of computed tomography increased as the first diagnostic technique (77.1%, 77.9%, and 84.2%, respectively; P=.001). In type A, surgical management increased (79.6%, 78.7%, and 84.5%; P=.045) and overall mortality decreased (41.2%, 34.5%, and 31.2%; P=.002), due to a reduction in surgical mortality (33.4%, 25.1%, and 23.9%; P=.003). In type B, endovascular treatment increased (22.8%, 32.8%, and 38.7%; P=.006), while medical and surgical treatment decreased. Overall type B mortality also decreased (21.6%, 16.1%, and 12.0%; P=.005) in line with a reduction in mortality with medical (16.8%, 13.8%, and 8.8%, P=.030) and endovascular (27.0%, 18.0%, and 9.2%; P=.009) treatments.
ConclusionsThe iterations of RESA show a decrease in mortality from type A AAS, coinciding with an increase in surgical treatment and a reduction in surgical mortality. In type B, the use of endovascular treatment was associated with improved survival, allowing better management in patients with complications.
Keywords
Acute aortic syndrome (AAS) is a cardiovascular emergency with high morbidity and mortality.1 According to recent studies, its annual incidence lies between 110 and 170 cases per 1 million population,2,3 which is considerably higher than previous estimates. Another recent study showed that approximately 1 in 2 patients with AAS dies before reaching a hospital.4 In-hospital mortality remains high (20%-35%),1 despite advances in diagnosis and treatment. A high degree of clinical suspicion, a well-defined diagnostic strategy, and a medical-surgical team with ample experience are crucial to improving survival in AAS.
Our understanding of clinical manifestations, complications, and treatment in AAS has improved significantly since the creation of the International Registry of Acute Aortic Dissection (IRAD).5 The IRAD, however, is a global registry of referral hospitals and its findings cannot be extrapolated to general clinical practice. A number of national AAS registries, each with different approaches and potential sources of bias, have appeared in recent years,6-9 but their findings do not accurately reflect the situation in Spain. The Spanish Registry of Acute Aortic Syndrome (RESA) was created to provide insights into AAS diagnosis, treatment, and mortality in Spain and to track changes over time. Most of the country's tertiary care hospitals participate in the registry, which contains data from 3 periods spanning 15 years: 2005-2006 (RESA I),10 2012-2013 (RESA II),11 and 2018-2019 (RESA III). In this time, advances in diagnostic imaging and surgical and endovascular techniques have improved the diagnosis and treatment of AAS.12
The aim of this study was to describe the results of RESA III and compare them to data from the full series to gain insights into changes in the diagnosis and management of AAS in the 15 years that RESA has been operating and to examine possible effects on mortality.
MethodsThe RESA is a prospective, protocol-based, observational, descriptive registry that contains AAS data supplied by tertiary care hospitals that provide around-the-clock cardiovascular and hemodynamic imaging and heart surgery services. Of the 34 hospitals invited to participate in the registry, 24 participated in RESA I,10 26 in RESA II11 and 30 in RESA III. The hospitals agreed to prospectively collect data on consecutive patients with AAS diagnosed at their hospital or referred from another hospital over a 2-year period. The inclusion periods were January 1, 2005 to December 31, 2006 for RESA I; January 1, 2012 to December 31, 2013 for RESA II; and January 1, 2018 to December 31, 2019 for RESA III. A total of 1902 patients were enrolled: 519 in RESA I,6 629 in RESA II,11 and 754 in RESA-III; 1328 (69.9%) had acute type A aortic dissection (ATAAD) and 574 (30.1%) had acute type B aortic dissection (ATBAD).
Data collectionData were collected and entered into the database using a prespecified system.10 In brief, each hospital was asked to appoint a coordinator responsible for collecting and verifying all the data on patients diagnosed with AAS during the study periods. Patients were identified by checking emergency and imaging department records and hospital discharge reports. The case report form included 140 variables on demographics, clinical history, physical examination and imaging findings, treatment, and in-hospital complications and mortality. The data were stored in a purpose-designed database built using the REDCap (Research Electronic Data Capture; Vanderbilt University Medical Center) software application. The study protocol complied with the principles of the 1975 Declaration of Helsinki and was approved by the ethics committees at Hospital Universitario Vall d’Hebron and the other participating hospitals. Informed consent was obtained from all patients. To facilitate comparisons, the study variables were defined using the same criteria as those used in the IRAD.1 When the IRAD was created in 1996, it included data from 24 hospitals in Europe, the United States, and Japan. This number has since grown to more than 60. Patients are considered to have a history of atherosclerosis if they have a previous diagnosis of ischemic heart disease, obliterative arteriopathy, or stroke. Shock is defined as a systolic blood pressure <80mmHg and impaired renal function as a creatinine level >1.5mg/100mL or a glomerular filtration rate <30/min/1.73 m2. All available data were analyzed for patients with missing data, none of whom were excluded. Doubts were resolved by telephoning the registry coordinator at the corresponding hospital. Patients with chronic, posttraumatic, or iatrogenic aortic dissection were not included in the study.
Data analysisData were analyzed in STATA version 15.1 (StataCorp, USA). We analyzed demographic information, initial diagnostic study, complications, treatment, and in-hospital mortality. Quantitative variables are expressed as mean±standard deviation and qualitative variables as a percentage. Between-group comparisons were made using analysis of variance for quantitative variables and the chi-square or Fisher exact test for qualitative variables. A nonparametric trend test was also performed. Statistical significance was set at a P value of less than .05.
ResultsIn total, 1902 patients with AAS were enrolled in the RESA during the 3 periods. Their mean age was 60.7±12.5 years and 74% were men. The demographic and clinical history data are summarized in table 1. The proportion of women diagnosed with AAS increased with time, rising from 22.5% in RESA I to 27.0% in RESA II and 29.6% in RESA III (P=.006). There was also an increase in the frequency of a past history of aortic aneurysm and aortic valve disease, which was particularly notable in RESA III.
Demographic characteristics of patients in the Spanish Registry of Acute Aortic Syndrome (RESA)
| Variable | Total (n=1902) | RESA I (n=519) | RESA II (n=629) | RESA III (n=754) | P | Trend, P |
|---|---|---|---|---|---|---|
| Age, y | 63.6±13.1 | 60.9±13.3 | 64.7±14.0 | 65.6±11.7 | .001 | .001 |
| Age >70 y | 590 (29.4) | 154 (29.7) | 238 (38.8) | 167 (37.7) | .001 | .036 |
| Male sex | 1392 (73.2) | 402 (77.5) | 459 (73.0) | 531 (70.4) | .020 | .006 |
| Hypertension | 1.335 (70.2) | 369 (71.1) | 410 (65.2) | 556 (73.7) | .061 | .172 |
| Smoker | 820 (43.1) | 233 (44.9) | 259 (41.2) | 328 (43.5) | .123 | .269 |
| Dyslipidemia | 537 (28.2) | 116 (22.4) | 190 (30.2) | 231 (30.6) | .009 | .088 |
| Diabetes mellitus | 178 (9.4) | 43 (8.3) | 56 (8.9) | 79 (10.5) | .489 | .275 |
| Ischemic heart disease | 173 (9.1) | 44 (8.5) | 53 (8.4) | 76 (10.1) | .545 | .413 |
| Atherosclerosis | 303 (15.9) | 97 (18.7) | 127 (20.2) | 79 (17.8) | .818 | .817 |
| Marfan syndrome | 65 (3.4) | 33 (6.4) | 14 (2.2) | 18 (2.4) | .001 | .001 |
| Aortic aneurysm | 406 (21.3) | 89 (17.1) | 118 (18.8) | 199 (26.4) | .001 | .001 |
| Aortic valve disease | 188 (9.9) | 46 (8.9) | 50 (7.9) | 92 (12.2) | .026 | .056 |
| Heart surgery | 120 (6.3) | 34 (6.6) | 39 (6.2) | 47 (6.2) | .322 | .139 |
Values are expressed as No. (%) or mean±standard deviation.
Computed tomography (CT) was the initial diagnostic study in all 3 periods and its use increased from 77.1% in RESA I to 77.9% in RESA II and 84.2% in RESA III; P=.001). ATAAD was more common than ATBAD (1328 cases [69.9%] vs 574 [30.1%]). The frequency of intramural hematoma and penetrating aortic ulcer was similar in the 3 periods. Time from symptom onset to AAS diagnosis became significantly shorter, with 70.3% of cases being diagnosed within 24hours in RESA I compared with 84.0% in RESA II and 86.5% in RESA III (P=.012). There were no significant differences in diagnostic delay within the first 24hours.
AAS complications diagnosed on admission (before surgical or endovascular treatment) were largely similar in the 3 periods, although in RESA III, there was a higher frequency of coma and peripheral ischemia and a lower frequency of visceral ischemia (table 2).
Diagnostic imaging techniques and complications according to the Spanish Registry of Acute Aortic Syndrome (RESA)
| Total (n=1902) | RESA I (n=519) | RESA II (n=629) | RESA III (n=754) | P | Trend, P | |
|---|---|---|---|---|---|---|
| Initial diagnostic imaging technique | ||||||
| Computed tomography | 1527 (80.3) | 400 (77.1) | 490 (77.9) | 637 (84.2) | .001 | .001 |
| Transesophageal echocardiogram | 52 (2.7) | 7 (13.5) | 19 (3.0) | 26 (3.4) | .001 | .001 |
| Transthoracic echocardiogram | 197 (10.4) | 24 (4.6) | 92 (14.6) | 81 (10.7) | .003 | .003 |
| Cardiac magnetic resonance imaging | 38 (1.9) | 2 (0.4) | 26 (4.1) | 10 (1.3) | .487 | .092 |
| Angiography | 25 (1.3) | 15 (2.9) | 2 (0.3) | 8 (1.1) | .745 | .345 |
| Diagnostic delays | ||||||
| <24h | 70.3 | 84.0 | 86.5 | .042 | .012 | |
| Median time to diagnosis within first 24h | 5 [3-10] | 5 [3-15] | 5 [3-11] | .874 | .748 | |
| Type of acute aortic dissection | ||||||
| Type A | 1329 (69.8) | 357 (68.8) | 443 (70.4) | 529 (70.3) | .731 | .630 |
| Type B | 573 (30.2) | 162 (31.2) | 186 (29.6) | 225 (29.7) | ||
| Dissection | 1.537 (80.8) | 430 (82.5) | 495 (78.9) | 612 (81.2) | .195 | .569 |
| Hematoma | 249 (13.1) | 64 (12.3) | 83 (13.1) | 102 (13.5) | .089 | .865 |
| Penetrating ulcer | 116 (6.1) | 25 (4.8) | 51 (8.1) | 40 (5.3) | .514 | .379 |
| Complications | ||||||
| Shock | 301 (15.9) | 97 (18.7) | 90 (14.3) | 114 (15.3) | .192 | .138 |
| Coma | 66 (3.5) | 15 (2.9) | 20 (3.2) | 31 (4.2) | .416 | .205 |
| Tamponade | 196 (10.3) | 59 (11.4) | 53 (8.4) | 84 (11.1) | .161 | .959 |
| Visceral ischemia | 108 (5.7) | 33 (6.4) | 38 (6.0) | 37 (4.9) | .040 | .360 |
| Peripheral ischemia | 202 (10.6) | 46 (8.9) | 64 (10.1) | 92 (12.2) | .018 | .084 |
| Periaortic hematoma | 469 (27.7) | 111 (31.4) | 169 (26.9) | 189 (25.6) | .120 | .063 |
| Kidney failure | 379 (19.9) | 94 (18.2) | 146 (19.1) | 139 (18.4) | .321 | .673 |
Values are expressed as No. (%) or median (interquartile range).
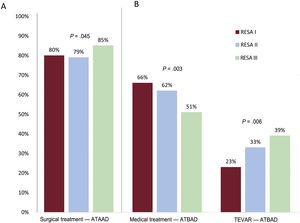
The proportion of ATAAD patients managed surgically increased from 79.6% in RESA I to 78.7% in RESA II and 84.5% in RESA III (P=.045) (figure 1A). There was also a significant increase in the age of these patients (58.6±12.6 years in RESA I, 61.1±13.3 years in RESA II, 62.5±10.9 years in RESA III; P=.001). Preoperative CT was performed in 94.4% of ATAAD patients. The reasons for not performing surgery in RESA III were death in the emergency room or before transfer to the operating room (32.7%), comorbidities (30.1%), an age >80 years (22.6%), intramural hematoma (8.7%), and patient refusal (6.7%). The proportion of patients with ATBAD receiving medical treatment only decreased over time. The reduction was particularly evident in RESA III, with a rate of 50.7% vs 62.3% for RESA II and 66.0% for RESA I (P=.003). As expected, the proportion of patients undergoing endovascular treatment increased from 22.8% in RESA 1 to 32.8% in RESA II and 38.7% in RESA III (P=.006) (figure 1B). No significant differences were observed for the surgical treatment of ATBAD (11.2% in RESA I, 4.8% in RESA II, 10.6% in RESA III; P=0.474). The indications for endovascular treatment or open surgery in patients with ATBAD in RESA III were signs of contained aortic rupture (21%), hemodynamic instability (20%), refractory hypertension (17%), severe aortic dilatation (11%), visceral or peripheral ischemia (10%), extended dissection (6%), and persistent pain (4%).
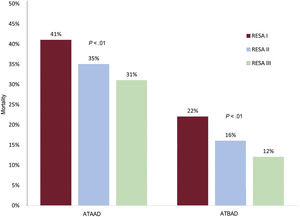
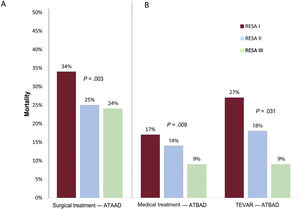
MortalityOverall in-hospital ATAAD mortality decreased from 41.2% in RESA I to 34.5% in RESA II and 31.2% in RESA III (P=.002) (table 3 and figure 2). Surgical mortality rates also declined over the periods (33.4%, 25.1%, 23.9%, respectively; P=.002) (figure 3A). Overall in-hospital mortality rates for ATBAD also dropped, from 22.8% in RESA I to 16.1% in RESA II and 12.0% in RESA III (P=.005) (figure 2). These decreases were accompanied by corresponding decreases in mortality among patients receiving medical treatment alone (16.8%, 13.8%, 8.8%; P=.030) and those treated with thoracic endovascular aortic repair (TEVAR) (27.0%, 18.0%, 9.2%; P=.009) (figure 3B). After adjustment for age and complications, the decrease in mortality rates in the ATAAD, ATBAAD, and treatment subgroups remained significant in RESA II and RESA III ().
Treatment of acute aortic syndrome and mortality in the Spanish Registry of Acute Aortic Syndrome (RESA)
| Type A | Type B | ||||||
|---|---|---|---|---|---|---|---|
| Total | Surgery | Medical treatment | Total | Medical treatment | TEVAR | Surgery | |
| Treatment | |||||||
| RESA I | 357 | 286 (79.6) | 71 (20.4) | 162 | 106 (65.4) | 41 (25.3) | 15(9.3) |
| RESA II | 443 | 347 (78.7) | 96 (21.3) | 186 | 116 (62.4) | 62 (33.3) | 8 (4.3) |
| RESA III | 529 | 447 (84.5) | 82 (15.5) | 225 | 114 (50.7) | 87 (38.7) | 24 (10.6) |
| Total | 1329 | 1080 (81.3) | 249 (18.7) | 573 | 336 (58.6) | 190 (33.2) | 47 (8.2) |
| P | — | 0.039 | — | — | 0.009 | 0.022 | 0.756 |
| Trend, P | — | 0.045 | — | — | 0.003 | 0.006 | 0.474 |
| Mortality | |||||||
| RESA I | 147 (41.2) | 95 (33.4) | 52 (71.2) | 37 (22.8) | 18 (16.8) | 10 (27.0) | 9 (50.0) |
| RESA II | 153 (34.5) | 87 (25.1) | 66 (68.7) | 30 (16.1) | 16 (13.8) | 11 (18.0) | 3 (33.3) |
| RESA III | 165 (31.2) | 107 (23.9) | 58 (70.3) | 27 (12.0) | 10 (8.8) | 8 (9.2) | 9 (37.5) |
| P | .007 | .003 | .913 | .018 | .094 | .031 | — |
| Trend, P | .002 | .002 | .675 | .005 | .030 | .009 | — |
TEVAR, thoracic endovascular aortic repair.
All values are expressed as No. (%), unless otherwise indicated.
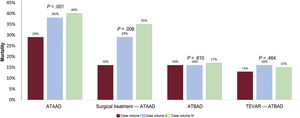
Annual volumes of AAS cases were associated with ATAAD but not ATBAD mortality. Only 7 hospitals had an annual volume of 12 or more ATAAD cases (volume IA hospitals) and mean mortality in this group was 28.8%, which is significantly lower than the rates of 38.1% observed in the 13 hospitals with an annual volume of 6 to 11 cases (volume IIA hospitals) and 40.4% in the 14 hospitals with an annual volume of fewer than 6 cases (volume IIIA hospitals) (P=.001). Mortality among ATAAD patients treated surgically was 16.6% in volume IA hospitals, 28.7% in volume IIA hospitals, and 34.9% in volume IIIA hospitals (P=.008). No significant differences were observed for ATBAD mortality according to case volume. The rates were 16.1% in hospitals with more than 6 ATBAD cases a year (volume IB hospitals), 15.6% in those with 3 to 6 cases a year (volume IIB hospitals), and 16.9% in those with fewer than 3 cases a year (volume IIIB hospitals) (P=.610). Mortality rates in ATBAD patients treated with TEVAR were similar in volume IB hospitals (13.4%), volume IIB hospitals (16.0%), and volume IIIB hospitals (15.1%) (P=.464) (figure 4).
Mortality rates according to annual volume of cases and procedures (surgery and thoracic endovascular aortic repair [TEVAR]) for acute type A aortic dissection (ATAAD) and acute type B aortic dissection. ATAAD case volume I: ≥ 12 cases or surgical interventions a year. ATAAD case volume II: 6-11 cases or surgical interventions a year. ATAAD case volume III: <6 cases or surgical interventions a year. ATBAD case volume I: ≥ 6 cases or TEVAR procedures a year. ATBAD case volume II: 3-5 cases or TEVAR procedures a year. ATBAD case volume III: <3 cases or TEVAR procedures a year.
Analysis of the Spanish AAS registry, RESA, shows that hospital deaths due to ATAAD and ATBAD dropped significantly over the 15-year period analyzed. The decrease was accompanied by an increase in the surgical treatment of ATAAD and the use of TEVAR for ATBAD (figure 5). Mortality also declined among ATAAD patients treated surgically and ATBAD patients who underwent TEVAR or received medical treatment only (figure 5). There was also an increase in patient age and the proportion of women in the registry over the 3 periods analyzed: RESA I (2005-2006), RESA II (2012-2013), and RESA III (2018-2019). Similar findings were reported in a recent population-based study.13 The proportion of patients diagnosed within 24hours of symptom onset decreased from 29.7% in RESA I to 13.5% in RESA III. Although diagnostic delays are not mentioned in most of the published series, early diagnosis could result in the enrollment of a higher proportion of patients with unstable disease or more complications.14,15 Our results are very similar to the delay of 4.3hours (range, 1.5-24hours) described for the IRAD series.1 Shorter times are probably difficult to achieve in a national registry of patients located at varying distances from their hospital. Fifteen years ago, no distinction was made between the indication of CT or transesophageal echocardiography in patients with suspected AAS.16 In our series, transesophageal echocardiography was the initial diagnostic test in 13.5% of patients in RESA I vs just 3.4% in RESA III. CT was the most common initial diagnostic test in our series, and its use increased from 77.1% in RESA I to 84.2% in RESA II. The main advantages of this imaging modality are diagnostic accuracy, possibility of assessing the entire aorta, speed, and availability. The European Society of Cardiology clinical practice guidelines17,18 recommend transthoracic echocardiography as the diagnostic test of choice in emergency departments, as it often provides a view of the myointimal septum in certain aortic segments, such as the root, arch, and abdominal aorta. It can also detect conditions such as pericardial effusion, aortic insufficiency, and ventricular dysfunction. Use of transthoracic echocardiography as the initial diagnostic test increased from 4.6% in RESA I to 10.7% in RESA III. This trend will probably continue, as this procedure, together with cardiac ultrasound, is being increasingly used in emergency departments.
Central illustration. A: number of patients with acute type A aortic dissection (ATAAD) and acute type B aortic dissection (ATBAD) during the 3 periods of the Spanish Registry of Acute Aortic Dissection (RESA). B: participating hospitals and geographic location. C: changes in overall ATAAD and ATBAD mortality. D: changes in ATAAD mortality after surgical treatment. E: changes in ATBAD mortality after medical treatment. F: changes in ATBAD mortality after thoracic endovascular aortic repair (TEVAR).
One of the most notable findings in RESA I was the high in-hospital mortality observed for both ATAAD and ATBAD. In the first case, 41% of patients died while in hospital compared with just 31% in RESA III. This significant reduction may have several explanations, including an increase in the proportion of ATAAD patients managed surgically (79.6% in RESA I vs 84.5% in RESA III) and a decrease in surgical mortality (33.4% vs 23.9%). ATAAD management and treatment has clearly improved in Spain, with hospitals implementing critical care protocols and accumulating surgical experience. In the IRAD series, surgical mortality in patients with ATAAD dropped from 25% to 18% over a similar period to that analyzed in the RESA. The IRAD, however, only contains data from referral hospitals that perform high volumes of aortic surgery. Its findings thus are not indicative of hospitals with low case loads. Surgical mortality rates in other national registries range from 17.0% to 23.1%.6,19 The first publication on the aortic dissection registry in China (Sino-RAD)8 described an overall mortality rate of 18.1% for ATAAD and a surgical mortality rate of just 5.1%. The registry contained data on 1002 patients with ATAAD treated over a 2-year period, but they were from just 15 hospitals, all large. The mean age of the patients (51 years) suggests possible inclusion bias, as patients in the RESA were on average 12 years older. A recent analysis of the Japanese Registry of Aortic Dissection (JRAD), which includes most of the country's hospitals, showed an overall mortality rate of just 11.8% among ATAAD patients, but 25% of these patients were not considered candidates for surgery.9
It is well known that ATBAD is associated with lower mortality than ATAAD. Medication is the treatment of choice for ATBAD in the absence of serious complications. Overall ATBAD mortality was 21% in RESA I, which is markedly higher than the rate of 15% described in the IRAD series. The rate of 12% reported for RESA III is much closer to the IRAD rate.1 One possible reason for decline in mortality among ATBAD patients might be the increase in the proportion of TEVAR procedures, which rose from 23% to 39%, and the associated decrease in mortality (from 27% to 9%). The sharp decline in mortality is probably due to accumulation of surgical experience, as TEVAR was still very new in RESA I. Although TEVAR is indicated for the treatment of patients with complications, such as imminent aortic rupture, severe hemodynamic instability, and visceral or peripheral ischemia, the criteria applied in RESA III were less strict and include aortic dilatation >45mm and refractory pain or blood pressure. The proportion of patients with ATBAD treated with medical management alone decreased from 66% in RESA I to 51% in RESA III, and the associated mortality fell from 17% to 9%. In a study of a selection of IRAD hospitals, 11% of patients with complicated ATBAD died after endovascular therapy.20 TEVAR now accounts for a considerable proportion of surgical treatments for ATBAD.21 Supporting previous findings for ATAAD,8,19,22 we observed a significant association between surgical mortality and hospital case volume, with significantly lower rates observed in hospitals performing at least 12 operations a year. These differences were not observed in relation to ATBAD or the number of cases treated with TEVAR, suggesting that endovascular therapy requires less experience and infrastructure than open surgery.
Although like all registry studies, this study has limitations, our findings are important as they are based on a large cohort of AAS patients treated at tertiary care hospitals in Spain and suggest that the reductions observed in in-hospital mortality over the 15-year period analyzed are not due to changes in patient profiles. A number of factors probably contributed to the better outcomes observed, including a higher degree of clinical suspicion, the implementation of diagnostic and medical treatment protocols, and increased experience with open surgery and TEVAR. Initiatives such as the Aortic Code project have also helped by improving the diagnosis of AAS, expediting the transfer of patients to referral centers, and building experience by creating a network of dedicated surgical teams.23
LimitationsOur findings cannot be extrapolated to general settings as the Spanish RESA registry only contains data from tertiary care hospitals. It could be argued that the improved survival outcomes observed among AAS patients over the 15-year study period are due to changes in patient profiles or a reduced incidence of serious complication. Visceral ischemia, for example, was less common in RESA III than in RESA I, but both peripheral ischemia and coma were more common. The frequency of other complications was similar over the 3 periods. The RESA III registry contained data from 30 hospitals, 6 more than in RESA I. We did not, however, detect any significant differences on excluding these hospitals. While the RESA contains data on demographics and cardiovascular history, it does not collect information on other conditions that could influence prognosis, such as chronic lung disease, chronic kidney failure, and cancer. Finally, the study design did not allow us to accurately identify diagnostic delays beyond 24hours for patients transferred from a non-RESA hospital.
ConclusionsIn-hospital ATAAD mortality in tertiary care Spanish hospitals decreased significantly from 2005 to 2019, and this decrease was accompanied by an increase in the surgical treatment of ATAAD and a decrease in associated mortality. ATBAD mortality rates also declined, probably because of an increased use of endovascular therapy, which precludes the need for open surgery, and a decrease in the use of medical management alone for patients with complications who are not eligible for surgery because of high surgical complexity and risk.
Advances in diagnostic and therapeutic approaches and surgical and endovascular techniques were associated with a significant decline in AAS mortality in Spain. Outcomes, however, are still far from optimal, and efforts must continue to improve the management of AAS and ensure the continuation of registries such as the RESA to help guide future directions.
FundingThe RESA registry was partly financed by the Spanish Society of Cardiology and Medtronic, which funded publication costs and the creation and maintenance of the database.
Authors’ ContributionsA. Evangelista Masip contributed to the study design and writing of the manuscript; A. López-Sainz to the writing and revision of the manuscript; A.J. Barros Membrilla, F. Calvo Iglesias, J. López Ayerbe, M. Azqueta Molluna, V.X. Mosquera Rodríguez, F Arregui Montoya, R. Tarrío Fernández, A. Revilla Orodea, V. Sánchez Sánchez, E.M. Cantero Pérez, C. Ferrera, D. Toral Sepúlveda, F. Nistal, and C. Fernández Golfín to the entry of patient data and revision of the manuscript; A. Sao to the statistical analysis; and J. Rodríguez-Palomares to the statistical analysis and revision of the manuscript.
Conflicts of InterestNone.
- –
AAS is an acute lesion of the aortic wall associated with high morbidity and mortality rates. It requires a high degree of clinical suspicion and appropriate diagnosis and treatment.
- –
Recent advances in diagnostic imaging and surgical and endovascular techniques have improved the diagnosis and treatment of AAS.
- –
Although national and international registries have added considerably to our understanding of AAS, their findings are difficult to apply in Spain due to differences in health care structures and their focus on referral hospitals.
- –
Comparison of the 3 periods analyzed in the Spanish AAS registry, RESA, showed an increase in patient age and the proportion of women enrolled. Diagnosis within 24hours of symptom onset improved over the 15-year study period.
- –
In-hospital ATAAD mortality in tertiary care Spanish hospitals decreased significantly from 2005 to 2019, and this decrease was accompanied by an increase in the surgical treatment of ATAAD and a decrease in associated mortality.
- –
ATBAD mortality rates also declined, probably because of an increase in the proportion of TEVAR procedures, reducing the need for open surgery, and better selection of patients for medical treatment alone.
We are grateful to the staff at the emergency, radiology, cardiac surgery, and vascular surgery departments at the participating hospitals for their invaluable contributions to the RESA over the years.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2022.01.017.




![Mortality rates according to annual volume of cases and procedures (surgery and thoracic endovascular aortic repair [TEVAR]) for acute type A aortic dissection (ATAAD) and acute type B aortic dissection. ATAAD case volume I: ≥ 12 cases or surgical interventions a year. ATAAD case volume II: 6-11 cases or surgical interventions a year. ATAAD case volume III: <6 cases or surgical interventions a year. ATBAD case volume I: ≥ 6 cases or TEVAR procedures a year. ATBAD case volume II: 3-5 cases or TEVAR procedures a year. ATBAD case volume III: <3 cases or TEVAR procedures a year. Mortality rates according to annual volume of cases and procedures (surgery and thoracic endovascular aortic repair [TEVAR]) for acute type A aortic dissection (ATAAD) and acute type B aortic dissection. ATAAD case volume I: ≥ 12 cases or surgical interventions a year. ATAAD case volume II: 6-11 cases or surgical interventions a year. ATAAD case volume III: <6 cases or surgical interventions a year. ATBAD case volume I: ≥ 6 cases or TEVAR procedures a year. ATBAD case volume II: 3-5 cases or TEVAR procedures a year. ATBAD case volume III: <3 cases or TEVAR procedures a year.](https://static.elsevier.es/multimedia/18855857/0000007500000010/v2_202212220558/S1885585722000706/v2_202212220558/en/main.assets/thumbnail/gr4.jpeg?xkr=eyJpdiI6IkxsYm1GZi9qcFpQN2tHYldlcTdDYnc9PSIsInZhbHVlIjoibjlCczl2WjUyYlI2cHYweXB6NmlWcHdGM0dYVjk2NE1ybkF4MmpObDVtTT0iLCJtYWMiOiIzMGY0Njk3YmJkZjgzNDBjNmE2YTM2MmIxMTNmZDkxYmM0ZjEyNWVjN2EzMmI1NTRkYzA4Y2IyMWUxZGZmNDE1IiwidGFnIjoiIn0=)
