Thyroid hormone affects the metabolism of all tissues in the body. The aim of this study was to analyze the prevalence and implications of thyroid disorders in a cohort of consecutive patients with spontaneous coronary artery dissection (SCAD).
MethodsA total of 73 patients with SCAD were analyzed. Baseline characteristics and clinical outcomes were compared between euthyroid and hypothyroid patients. Subsequently, the prevalence of thyroid function abnormalities and the clinical characteristics of SCAD patients were compared with those in 73 patients with acute coronary syndrome but without SCAD, matched by age, sex, and presentation.
ResultsMean age was 55 ± 12 years and 26% had hypothyroidism. Compared with patients with normal thyroid function, patients with SCAD and hypothyroidism were all women (100% vs 69%, P = .01), more frequently had dissection in distal (74% vs 41%, P = .03) and tortuous coronary segments (68% vs 41%, p = .03), and more frequently received conservative medical management (79% vs 41%, P = .007). During a mean clinical follow-up of 4.1 ± 3.8 years, 23% of the patients had adverse cardiac events irrespective of thyroid function status. The prevalence of hypothyroidism was higher in patients with SCAD than in matched patients with acute coronary syndrome without SCAD (26% vs 8%, P = .004).
ConclusionsThere is a high prevalence of hypothyroidism in patients with SCAD. Patients with SCAD and hypothyroidism are more frequently women, more frequently have distal dissections in tortuous vessels, and are more frequently managed with a conservative medical strategy.
Keywords
Spontaneous coronary artery dissection (SCAD) is an infrequent cause of coronary heart disease. Although more than 8 decades have passed since its first description,1 its pathophysiology and management are still a matter of debate.1 Its clinical presentation is variable: it usually presents as acute coronary syndrome (ACS), but some patients present with cardiogenic shock or even sudden cardiac death.2–7 SCAD is an especially important cause of ACS in young women without coronary risk factors,5 but most SCADs occur in middle-aged patients with cardiovascular risk factors.2–8
This entity has been linked to numerous factors or situations that can act as predisposing or triggering factors, including pregnancy, peripartum and perimenopausal statuses, coronary spasm, strenuous physical exercise, emotional stress, connective tissue diseases, and contraceptive use,9 recreational drugs (particularly cocaine), and vasoconstrictive substances (eg, antimigraine medication). SCAD has also been associated with inflammatory and autoimmune diseases,2–8,10 although inflammatory or autoimmune markers are rarely detected upon systematic analysis of their presence.11 Recently, fibromuscular dysplasia (FMD) has been identified as an emerging risk factor for SCAD, with a prevalence of up to 72% in some series.4,8
Thyroid hormones affect the metabolism of all cells and tissues in the body. The association of hypothyroidism with “noncoronary” spontaneous arterial dissections (principally aortic, carotid, and vertebral) is well known, reaching a prevalence of 31% in some studies.12 Although a patient with SCAD associated with hypothyroidism has been anecdotally described, the possible association and implications of hypothyroidism for patients with SCAD have not been systematically studied.13
The aim of this work was to analyze the prevalence of thyroid disorders in a cohort of consecutive patients with SCAD and to compare the clinical, anatomical, and prognostic characteristics according to the presence of a thyroid function disorder.
METHODSAll patients diagnosed with SCAD admitted to 2 tertiary university centers were included in this study. The first center, the Juan Ramón Jiménez University Hospital of Huelva, included patients from 2000 to 2017, whereas the second, the Princess University Hospital of Madrid, included them from 2010 to 2017. These 2 centers followed a similar protocol for the diagnosis of SCAD that was based on a high level of clinical suspicion for the presence of this disease. Two experienced interventional cardiologists with interest in this entity confirmed the angiographic diagnosis of SCAD in all patients after jointly reviewing the angiographic findings. In accordance with the classification of Saw et al.,14 patients were categorized as having type 1 dissection (showing an intimal flap on imaging and a double lumen with or without contrast staining), type 2 (showing extensive and diffuse narrowing of the arterial lumen) without an arterial flap (representing an intramural hematoma), and type 3 (showing a focal or tubular lesion mimicking an atherosclerotic lesion). The presence of coronary tortuosity was evaluated; its most extreme grade was classified as a “corkscrew” artery and its location in the vessel was defined according to the definitions proposed by the Mayo Clinic.15 An intracoronary imaging technique (intracoronary ultrasound or optical coherence tomography) was attempted in both centers to confirm or rule out the diagnosis in doubtful cases. Affected angiographic segments were measured using quantitative coronary angiography. In the final 5 years of the study, both centers also attempted to systematically evaluate the presence of FMD, although a specific diagnostic protocol was not followed. Only the classic multifocal diffuse “string-of-beads” pattern was defined as FMD.
All epidemiological data, risk factors, possible predisposing and triggering factors, clinical characteristics, treatment approaches, prognoses, and long-term onset of recurrence or major cardiac adverse events (MACE) were prospectively analyzed. Patients with clinical suspicion of a systemic disease classically associated with SCAD were studied according to standard clinical practice to try to confirm the diagnosis.2–8,10
Thyroid status was specifically analyzed and classified into 3 types: a) hypothyroidism if there was a previous clinical diagnosis or high thyrotropin concentrations were detected (TSH ≥ 5.0 mU/mL); b) euthyroidism when there was no history or previous diagnosis of altered thyroid function and the levels of TSH and free thyroxine (free T4) were normal; and c) hyperthyroidism if there was a history of this condition or the TSH level was ≤ 0.3 mU/mL. In turn, hypothyroid patients were divided into subgroups according to whether they received hormone replacement therapy and, if appropriate, whether they maintained good (0.3 mU/mL ≤ TSH 0.5 mU/mL) or poor (TSH ≥ 5.0 mU/mL) metabolic control. Thyroid hormone levels were prospectively determined as part of the study protocol for patients with ACS.
After hospital discharge, all patients with SCAD were prospectively followed up in the cardiology clinic to detect the occurrence of MACE (cardiovascular and all-cause death, acute myocardial infarction, stroke, need for coronary artery bypass surgery, and need for target lesion revascularization).
The protocol for the systematic study of SCAD has been evolving with improved understanding of the condition. The study protocol was presented in 2015 at the annual meeting of the Cardiac Catheterization and Interventional Cardiology Section of the Spanish Society of Cardiology, and the corresponding ethics committees of both hospitals approved its latest version in 2017.
In addition, and simply as a reference, a control group of consecutive patients with ACS but without SCAD was retrospectively selected from the electronic database of the Catheterization Service (Greco Management Software 2000); these patients were first matched with the SCAD patient series according to age, sex, and clinical presentation. A single experienced cardiologist reviewed all of the angiograms of this group. All patients with ACS underwent, by protocol, an extensive general analytical determination that included the thyroid profile.
Statistical AnalysisDiscrete variables are expressed as number and percentage and were compared with the chi-square test or Fisher exact test (if the expected values in the cells were < 5). Continuous variables are expressed as mean ± standard deviation and were compared using the Student t test. Nonnormally distributed continuous variables are expressed as median [interquartile range]. In the case of asymmetric distributions, a nonparametric test (Mann-Whitney U) was used. To evaluate differences between the euthyroid and hypothyroid SCAD groups, we used a proportional hazard model with Cox regression for the analysis of adverse events occurring during follow-up (expressed as hazard ratios [HRs] with 95% confidence intervals [95%CIs]). To assess associations for dichotomous variables between the SCAD and non-SCAD groups, the likelihood ratio or odds ratio was calculated. In addition, a conditional logistic analysis was performed to analyze the prevalence of hypothyroidism between the 2 series after adjustment for possible baseline confounding factors. The statistical analysis was performed using SPSS 22 statistical software. Statistical significance was set at P < .05.
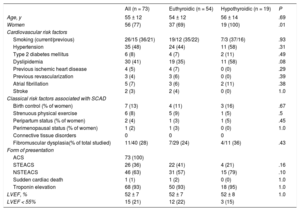
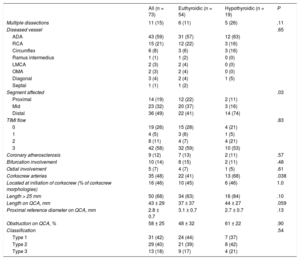
RESULTSThe study included 73 consecutive patients with SCAD; 26% of them were classified as having hypothyroidism. Most patients with SCAD were women (77%; n = 56), Caucasian, and with relatively few cardiovascular risk factors. The differences in the baseline characteristics of patients with SCAD according to their thyroid status are shown in Table 1. Most patients with SCAD and hypothyroidism received thyroid hormone replacement therapy (74%, n = 14) and maintained good metabolic control (86%, n = 12). Antithyroid antibodies were elevated in 16% (n = 3). All hypothyroid patients with SCAD were female (100% vs 69%; P = .01), with a nonsignificant tendency for higher prevalences of FMD (36% [4/11] vs 24% [7/29]; P = .43) and dyslipidemia (58% [n = 11] vs 35% [n = 24]; P = .09) vs euthyroid patients. In all patients, the form of presentation was ACS (Table 1). The angiographic characteristics of the 2 groups are compared in Table 2. Most patients had a single long (> 25mm) type 1 or 2 dissection located in the anterior descending artery and in distal coronary segments. SCAD patients with hypothyroidism had a higher frequency of distal vessel involvement (74% [n = 14] vs 41% [n = 22]; P = .03) and tended to show a more diffuse involvement (84% [n = 16] vs 63% [n = 34]; P = .1). Via quantitative coronary angiography measurement, the vessels of patients with SCAD tended to be smaller (mean proximal reference diameter on quantitative coronary angiography, 2.7 ± 0.7mm vs 3.1 ± 0.7mm; P = .13) and with more diffuse involvement (length on quantitative coronary angiography, 44 ± 27mm vs 37 ± 37mm; P = .059).
Clinical and Demographic Characteristics of the Spontaneous Coronary Artery Dissection Group
| All (n = 73) | Euthyroidic (n = 54) | Hypothyroidic (n = 19) | P | |
|---|---|---|---|---|
| Age, y | 55 ± 12 | 54 ± 12 | 56 ± 14 | .69 |
| Women | 56 (77) | 37 (69) | 19 (100) | .01 |
| Cardiovascular risk factors | ||||
| Smoking (current/previous) | 26/15 (36/21) | 19/12 (35/22) | 7/3 (37/16) | .93 |
| Hypertension | 35 (48) | 24 (44) | 11 (58) | .31 |
| Type 2 diabetes mellitus | 6 (8) | 4 (7) | 2 (11) | .49 |
| Dyslipidemia | 30 (41) | 19 (35) | 11 (58) | .08 |
| Previous ischemic heart disease | 4 (5) | 4 (7) | 0 (0) | .29 |
| Previous revascularization | 3 (4) | 3 (6) | 0 (0) | .39 |
| Atrial fibrillation | 5 (7) | 3 (6) | 2 (11) | .38 |
| Stroke | 2 (3) | 2 (4) | 0 (0) | 1.0 |
| Classical risk factors associated with SCAD | ||||
| Birth control (% of women) | 7 (13) | 4 (11) | 3 (16) | .67 |
| Strenuous physical exercise | 6 (8) | 5 (9) | 1 (5) | .5 |
| Peripartum status (% of women) | 2 (4) | 1 (3) | 1 (5) | .45 |
| Perimenopausal status (% of women) | 1 (2) | 1 (3) | 0 (0) | 1.0 |
| Connective tissue disorders | 0 | 0 | 0 | |
| Fibromuscular dysplasia(% of total studied) | 11/40 (28) | 7/29 (24) | 4/11 (36) | .43 |
| Form of presentation | ||||
| ACS | 73 (100) | |||
| STEACS | 26 (36) | 22 (41) | 4 (21) | .16 |
| NSTEACS | 46 (63) | 31 (57) | 15 (79) | .10 |
| Sudden cardiac death | 1 (1) | 1 (2) | 0 (0) | 1.0 |
| Troponin elevation | 68 (93) | 50 (93) | 18 (95) | 1.0 |
| LVEF, % | 52 ± 7 | 52 ± 7 | 52 ± 8 | 1.0 |
| LVEF < 55% | 15 (21) | 12 (22) | 3 (15) | |
ACS, acute coronary syndrome; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; SCAD, spontaneous coronary artery dissection; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, the data represent No. (%) or mean ± standard deviation.
Angiographic Characteristics of the Spontaneous Coronary Artery Dissection Group
| All (n = 73) | Euthyroidic (n = 54) | Hypothyroidic (n = 19) | P | |
|---|---|---|---|---|
| Multiple dissections | 11 (15) | 6 (11) | 5 (26) | .11 |
| Diseased vessel | .65 | |||
| ADA | 43 (59) | 31 (57) | 12 (63) | |
| RCA | 15 (21) | 12 (22) | 3 (16) | |
| Circumflex | 6 (8) | 3 (6) | 3 (16) | |
| Ramus intermedius | 1 (1) | 1 (2) | 0 (0) | |
| LMCA | 2 (3) | 2 (4) | 0 (0) | |
| OMA | 2 (3) | 2 (4) | 0 (0) | |
| Diagonal | 3 (4) | 2 (4) | 1 (5) | |
| Septal | 1 (1) | 1 (2) | ||
| Segment affected | .03 | |||
| Proximal | 14 (19) | 12 (22) | 2 (11) | |
| Mid | 23 (32) | 20 (37) | 3 (16) | |
| Distal | 36 (49) | 22 (41) | 14 (74) | |
| TIMI flow | .83 | |||
| 0 | 19 (26) | 15 (28) | 4 (21) | |
| 1 | 4 (5) | 3 (6) | 1 (5) | |
| 2 | 8 (11) | 4 (7) | 4 (21) | |
| 3 | 42 (58) | 32 (59) | 10 (53) | |
| Coronary atherosclerosis | 9 (12) | 7 (13) | 2 (11) | .57 |
| Bifurcation involvement | 10 (14) | 8 (15) | 2 (11) | .48 |
| Ostial involvement | 5 (7) | 4 (7) | 1 (5) | .61 |
| Corkscrew arteries | 35 (48) | 22 (41) | 13 (68) | .038 |
| Located at initiation of corkscrew (% of corkscrew morphologies) | 16 (46) | 10 (45) | 6 (46) | 1.0 |
| Length > 25 mm | 50 (68) | 34 (63) | 16 (84) | .10 |
| Length on QCA, mm | 43 ± 29 | 37 ± 37 | 44 ± 27 | .059 |
| Proximal reference diameter on QCA, mm | 2.8 ± 0.7 | 3.1 ± 0.7 | 2.7 ± 0.7 | .13 |
| Obstruction on QCA, % | 58 ± 25 | 48 ± 32 | 61 ± 22 | .90 |
| Classification | .54 | |||
| Type 1 | 31 (42) | 24 (44) | 7 (37) | |
| Type 2 | 29 (40) | 21 (39) | 8 (42) | |
| Type 3 | 13 (18) | 9 (17) | 4 (21) | |
ADA, anterior descending artery; LMCA, left main coronary artery; OMA, obtuse marginal artery; QCA, quantitative coronary angiography; RCA, right coronary artery; TIMI, Thrombolysis In Myocardial Infarction.
Values represent No. (%) or mean ± standard deviation.
Half of the patients had”corkscrew“coronary arteries and, in half of these arteries, the dissections began at the beginning of the first curvature of the vessel (Table 2). The group with hypothyroidism showed a higher prevalence of arteries with corkscrew morphology (68% [n = 13] vs 41% [n = 22]; P = .038). In 2 patients, both with hypothyroidism, the diagnostic catheter caused an iatrogenic coronary artery dissection in apparently healthy proximal segments of the same artery that had a distal SCAD.
Intracoronary imaging techniques were used in one-third of patients (Table 2). During the first years, because it had been available for more than a decade, intracoronary ultrasound was used more often than optical coherence tomography, a trend that was reversed in recent years. These techniques were used at the discretion of the cardiologist performing the coronary angiography (mainly in patients with type 2 or 3 dissections) to confirm the diagnosis or optimize patient management.
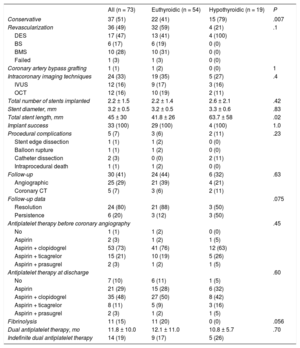
The therapeutic management of patients with SCAD is summarized in Table 3. Percutaneous coronary intervention was performed in half of the patients; 64% (n = 23) of these patients received a drug-eluting stent. Percutaneous coronary intervention was used primarily for patients diagnosed more than 10 years earlier, and conservative medical management was more frequent in more recently diagnosed patients (27% were conservatively managed before 2011 vs 61% after; P = .008). Only 1 patient who had a concomitant aortic dissection required coronary artery bypass surgery. Notably, a more conservative medical treatment strategy was chosen (79% [n = 15] vs 41% [n = 22]; P = .007) in the SCAD group with hypothyroidism (with a more distal and diffuse involvement and somewhat smaller and more tortuous vessels). Illustrative examples from this series are shown in Figure 1 and Figure 2.
Management of the Spontaneous Coronary Artery Dissection Group
| All (n = 73) | Euthyroidic (n = 54) | Hypothyroidic (n = 19) | P | |
|---|---|---|---|---|
| Conservative | 37 (51) | 22 (41) | 15 (79) | .007 |
| Revascularization | 36 (49) | 32 (59) | 4 (21) | .1 |
| DES | 17 (47) | 13 (41) | 4 (100) | |
| BS | 6 (17) | 6 (19) | 0 (0) | |
| BMS | 10 (28) | 10 (31) | 0 (0) | |
| Failed | 1 (3) | 1 (3) | 0 (0) | |
| Coronary artery bypass grafting | 1 (1) | 1 (2) | 0 (0) | 1 |
| Intracoronary imaging techniques | 24 (33) | 19 (35) | 5 (27) | .4 |
| IVUS | 12 (16) | 9 (17) | 3 (16) | |
| OCT | 12 (16) | 10 (19) | 2 (11) | |
| Total number of stents implanted | 2.2 ± 1.5 | 2.2 ± 1.4 | 2.6 ± 2.1 | .42 |
| Stent diameter, mm | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.3 ± 0.6 | .83 |
| Total stent length, mm | 45 ± 30 | 41.8 ± 26 | 63.7 ± 58 | .02 |
| Implant success | 33 (100) | 29 (100) | 4 (100) | 1.0 |
| Procedural complications | 5 (7) | 3 (6) | 2 (11) | .23 |
| Stent edge dissection | 1 (1) | 1 (2) | 0 (0) | |
| Balloon rupture | 1 (1) | 1 (2) | 0 (0) | |
| Catheter dissection | 2 (3) | 0 (0) | 2 (11) | |
| Intraprocedural death | 1 (1) | 1 (2) | 0 (0) | |
| Follow-up | 30 (41) | 24 (44) | 6 (32) | .63 |
| Angiographic | 25 (29) | 21 (39) | 4 (21) | |
| Coronary CT | 5 (7) | 3 (6) | 2 (11) | |
| Follow-up data | .075 | |||
| Resolution | 24 (80) | 21 (88) | 3 (50) | |
| Persistence | 6 (20) | 3 (12) | 3 (50) | |
| Antiplatelet therapy before coronary angiography | .45 | |||
| No | 1 (1) | 1 (2) | 0 (0) | |
| Aspirin | 2 (3) | 1 (2) | 1 (5) | |
| Aspirin + clopidogrel | 53 (73) | 41 (76) | 12 (63) | |
| Aspirin + ticagrelor | 15 (21) | 10 (19) | 5 (26) | |
| Aspirin + prasugrel | 2 (3) | 1 (2) | 1 (5) | |
| Antiplatelet therapy at discharge | .60 | |||
| No | 7 (10) | 6 (11) | 1 (5) | |
| Aspirin | 21 (29) | 15 (28) | 6 (32) | |
| Aspirin + clopidogrel | 35 (48) | 27 (50) | 8 (42) | |
| Aspirin + ticagrelor | 8 (11) | 5 (9) | 3 (16) | |
| Aspirin + prasugrel | 2 (3) | 1 (2) | 1 (5) | |
| Fibrinolysis | 11 (15) | 11 (20) | 0 (0) | .056 |
| Dual antiplatelet therapy, mo | 11.8 ± 10.0 | 12.1 ± 11.0 | 10.8 ± 5.7 | .70 |
| Indefinite dual antiplatelet therapy | 14 (19) | 9 (17) | 5 (26) | |
BMS, bare-metal stent; BS, bioabsorbable stent; CT, computed tomography; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Values represent No. (%) or mean ± standard deviation.
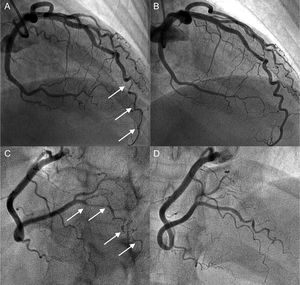
Coronary angiogram in a hypothyroid patient with multiple spontaneous coronary artery dissections. A and B: angiogram of the anterior descending artery with type 2 dissection (intramural hematoma) with involvement of a distal corkscrew segment (arrows) and its resolution during follow-up. C and D: angiogram of the right posterolateral branch with type 2 dissection (arrows) and its resolution during follow-up.
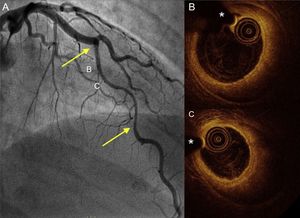
Coronary angiography and OCT imaging in a hypothyroid patient with spontaneous coronary artery dissection. A: angiogram of the anterior descending artery with a large type 2 dissection (arrows). B and C: OCT images of the proximal and mid segments showing a complete intimomedial flap separating the false lumen (large intramural hematoma) from the real one, where the OCT catheter and guidewire are located. OCT, optical coherence tomography. *Wire artifact.
At hospital discharge, dual antiplatelet therapy was maintained in 71% of patients. Two patients died during hospitalization; in the rest, once the acute phase was over, the symptoms resolved and the prognosis was favorable.
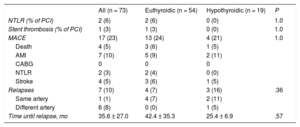
Long-term clinical follow-up was performed in all patients (4.1 ± 3.8 years; median, 3.1 [1.0-6.7] years) with a general MACE rate of 23% (n = 17) (Table 4), mainly due to revascularization and reinfarction. Fibrinolysis, previous anticoagulation, the type of conservative or interventional treatment, and corkscrew arteries were not associated with an increased long-term risk of MACE. No differences were found in the clinical course of patients with SCAD according to thyroid function status (HR = 0.76, 95%CI, 0.23-2.47; P = .65). Seven patients (10%) developed SCAD recurrence during follow-up, generally in a different artery from the first one (all of these patients received beta-blocker treatment). Hypothyroid patients had an SCAD recurrence rate similar to that of euthyroid patients (HR, 0.22, 95%CI, 0.38-1.35; P = .1).
Events During Follow-up in the Spontaneous Coronary Artery Dissection Group
| All (n = 73) | Euthyroidic (n = 54) | Hypothyroidic (n = 19) | P | |
|---|---|---|---|---|
| NTLR (% of PCI) | 2 (6) | 2 (6) | 0 (0) | 1.0 |
| Stent thrombosis (% of PCI) | 1 (3) | 1 (3) | 0 (0) | 1.0 |
| MACE | 17 (23) | 13 (24) | 4 (21) | 1.0 |
| Death | 4 (5) | 3 (6) | 1 (5) | |
| AMI | 7 (10) | 5 (9) | 2 (11) | |
| CABG | 0 | 0 | 0 | |
| NTLR | 2 (3) | 2 (4) | 0 (0) | |
| Stroke | 4 (5) | 3 (6) | 1 (5) | |
| Relapses | 7 (10) | 4 (7) | 3 (16) | .36 |
| Same artery | 1 (1) | 4 (7) | 2 (11) | |
| Different artery | 6 (8) | 0 (0) | 1 (5) | |
| Time until relapse, mo | 35.6 ± 27.0 | 42.4 ± 35.3 | 25.4 ± 6.9 | .57 |
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; MACE, major adverse cardiovascular events; NTLR, need for target lesion revascularization; PCI, percutaneous coronary intervention.
Unless otherwise indicated, the data represent No. (%) or mean ± standard deviation.
A late anatomical review of the changes over time in SCAD morphology (on angiography or coronary computed tomography) was performed in 41% of the patients (n = 30) (median follow-up time, 2.5 [1.1-5.0] years). In most patients (80%), the SCAD disappeared during follow-up imaging. However, the SCAD persisted in 6 patients (mainly patients who, at baseline, had type 1 spiral dissections that affected long coronary vessels). There was a nonsignificant tendency for less symptom resolution in hypothyroid patients than in euthyroid patients (50% [n = 3] vs 12% [n = 3]; P = .08).
Finally, simply as a reference, the characteristics of the 73 patients with SCAD were compared with those of the control group of 73 consecutive patients with ACS (matched for age, sex, and clinical presentation) but without SCAD (Table 5). The SCAD group had a significantly higher prevalence of hypothyroidism than the ACS patients without SCAD (26% [n = 19] vs 8% [n = 6]; P = .004; odds ratio = 3.92; 95%CI, 1.46-10.52). Compared with ACS patients without SCAD, patients with SCAD had a significantly lower prevalence of classic cardiovascular risk factors, mainly corkscrew arteries, with greater distal involvement and involvement of the anterior descending artery (Table 5).
Baseline Characteristics of the Groups With Spontaneous Coronary Artery Dissection and With ACS Without Spontaneous Coronary Artery Dissection
| SCAD (n = 73) | Controls (n = 73) | OR (95%CI) | P | |
|---|---|---|---|---|
| Age, y | 55 ± 12 | 55 ± 12 | .88 | |
| Women | 56 (77) | 56 (77) | 1 | |
| Cardiovascular risk factors | ||||
| Smoking | 41 (57) | 56 (77) | 0.389 (0.19-0.79) | .009 |
| Current | 26 (36) | 40 (55) | 0.45 (0.23-0.89) | .02 |
| Previous | 15 (21) | 16 (22) | 1.08 (0.49-2.40) | .84 |
| Hypertension | 35 (48) | 46 (63) | 0.54 (0.27-1.04) | .068 |
| Type 2 diabetes mellitus | 6 (8) | 22 (30) | 0.20 (0.07-0.54) | .001 |
| Dyslipidemia | 30 (41) | 40 (55) | 0.57 (0.29-1.10) | .09 |
| Previous ischemic heart disease | 4 (5) | 7 (10) | 0.54 (0.15-1.95) | .25 |
| Previous revascularization | 3 (4) | 6 (8) | 0.47 (0.11-1.99) | .24 |
| Atrial fibrillation | 5 (7) | 2 (3) | 2.6 (0.48-13.9) | .22 |
| Stroke | 2 (3) | 1 (1) | 2.02 (0.17-22.8) | .55 |
| Classical risk factors associated with SCAD | ||||
| Birth control (% of women) | 7 (13) | 1 (2) | 7.85 (0.93-66.1) | .06 |
| Strenuous physical exercise | 6 (8) | 0 (0) | .015 | |
| Peripartum status (% of women) | 2 (4) | 0 (0) | .25 | |
| Perimenopausal status (% of women) | 1 (3) | 1 (3) | 1.0 (0.06-16.39) | 1.0 |
| Connective tissue disorders | 0 | 0 | ||
| Thyroid function alteration | 19 (26) | 8 (11) | .019 | |
| Hypothyroidism | 19 (26) | 6 (8) | 2.85 (1.16-7.04) | .004 |
| Hyperthyroidism | 0 (0) | 2 (3) | 3.92 (1.46-10.52) | — |
| Acute coronary syndrome | 73 (100) | 73 (100) | 1.0 | |
| STEACS | 26 (36) | 35 (48) | 0.60 (0.30-1.16) | .13 |
| NSTEACS | 46 (63) | 37 (51) | 1.65 (0.85-3.20) | .13 |
| Sudden cardiac death | 1 (1) | 1 (1) | 1.0 | |
| Diseased vessel | ||||
| ADA | 47 (64) | 31 (42) | 2.44 (1.25-4.77) | .008 |
| RCA | 15 (21) | 23 (32) | 0.56 (0.26-1.19) | .13 |
| Circumflex | 9 (12) | 9 (12) | 1.0 (0.37-2.68) | 1.0 |
| LMCA | 2 (3) | 10 (14) | 0.17 (0.04-0.84) | .016 |
| Segment affected | ||||
| Proximal | 14 (19) | 27 (37) | 0.40 (0.19-0.85) | .016 |
| Mid | 23 (32) | 31 (43) | 0.62 (0.32-1.22) | .17 |
| Distal | 36 (49) | 15 (21) | 3.76 (1.81-7.80) | .0002 |
| TIMI flow | ||||
| 0 | 19 (26) | 17 (24) | 0.60 (0.30-1.16) | .69 |
| 1 | 4 (5) | 2 (3) | 2.06 (0.36-11.6) | .68 |
| 2 | 8 (11) | 9 (13) | 0.88 (0.32-2.41) | .79 |
| 3 | 42 (58) | 44 (61) | 0.89 (0.46-1.72) | .74 |
| Corkscrew arteries | 35 (48) | 20 (27) | 2.44 (1.22-4.86) | .01 |
95%CI, 95% confidence interval; ACS, acute coronary syndrome; ADA, anterior descending artery; LMCA, left main coronary artery; NSTEACS, non-ST-segment elevation acute coronary syndrome; OR, odds ratio; RCA, right coronary artery; SCAD, spontaneous coronary artery dissection; STEACS, ST-segment elevation acute coronary syndrome; TIMI, Thrombolysis In Myocardial Infarction.
Values represent No. (%) or mean ± standard deviation.
The present work is the first to specifically analyze the possible association and implications of hypothyroidism in patients with SCAD. The study analyzed all consecutive patients diagnosed with SCAD (n = 73) in 2 tertiary hospitals over a long period. This is the largest series of this disease in Spain. Patients with SCAD had an elevated prevalence of hypothyroidism. The findings indicate a possible association between these 2 entities. However, association does not necessarily imply causation. A comparison of patients with SCAD according to their thyroid function status revealed that patients with SCAD and hypothyroidism were more likely to be women and have more distal dissections and dissections in corkscrew arteries and to be treated more conservatively than euthyroid patients with SCAD. Via an exploratory approach, we found a lower prevalence of thyroid alterations in the group of ACS patients without SCAD who had been matched by age, sex, and form of presentation. These data indicate that the prevalence of thyroid disorders is higher in patients with ACS caused by SCAD.
SCAD is a rare disease that remains underdiagnosed. However, the interest in and concern about this entity and the availability of new diagnostic techniques explain its growing diagnosis. Thyroid hormones play an essential role in the function of the cardiovascular system and cardiac hemodynamics.16–18 Even slight changes in thyroid function affect heart rate and rhythm, ventricular function, and circulating cholesterol and increase the risk of coronary heart disease and mortality.19 Although it is still a matter of debate,20 largely because of the different definitions of hypothyroidism,21 hypothyroidism is found in 1% to 2% of populations without iodine deficiency and is up to 10 times more frequent in women than in men.22
Recent studies have also shown a relatively high prevalence of thyroid disorders in patients with ACS.23 Zhang et al.24 studied the relationship between hypothyroidism and MACE in 2430 individuals who underwent percutaneous coronary intervention (half of whom had ACS). Hypothyroid patients had a higher rate of MACE than euthyroid patients. In addition, an adequate management of the condition by hormone replacement therapy effectively prevented events. However, none of these studies mentioned the percentage of ACS due to SCAD.
A relationship has already been shown between thyroid disease and”noncoronary“arterial dissection in different territories. On the one hand, a number of series indicate an association between thyroid alterations and aortic dissections. In a series of 101 patients with aortic dissection, Rosenmann et al.12 determined a 22% prevalence of hypothyroidism vs 8% in the control group. This increased prevalence could be explained by an altered metabolism of glycosaminoglycans in both hypothyroidism and aortic dissection.12 On the other hand, hypothyroidism has been associated with a higher frequency of iatrogenic coronary artery dissection during angioplasty.25 In the present series, 2 hypothyroid patients developed an intraprocedural iatrogenic coronary artery dissection in a previously angiographically healthy segment. Previous studies had already indicated an increased risk of iatrogenic dissection in apparently healthy segments of patients with SCAD.26
Finally, thyroid alterations have also been associated with strokes caused by spontaneous cervical artery dissection. Pezzini et al.27 compared 29 patients with stroke secondary to cervical artery dissection vs 29 with stroke without dissection. Analysis of thyroid autoimmunity parameters revealed that the cervical artery dissection group was more likely to show antithyroid antibodies.27 This form of autoimmunity could be related to the local inflammatory process and to the cervical artery dissection. A recent meta-analysis confirmed this association in patients younger than 65 years of age.28
Interestingly, despite multiple studies linking arterial dissections at different levels to the presence of thyroid alterations, the relationship between hypothyroidism and SCAD has only been indicated in the literature in very anecdotal or circumstantial cases. Its presence has been mentioned in the general description of possible risk factors and comorbidities, with prevalences of 11.9% and 13.1%.8,29 However, none of these series has specifically analyzed this possible association, merely marginally mentioning the link. Only 1 case of SCAD in a patient with severe hypothyroidism13 and another with multivessel disease during the puerperium30 have been described in detail.
The underlying pathophysiology possibly explaining the association between hypothyroidism and SCAD is currently unknown. Although there are no data on specific histological changes in the coronary wall, myxedema may be involved.31 Thus, interstitial retention of water and sodium in the vascular wall has been described in hypothyroidism, with the deposition of hydrophilic mucopolysaccharides, an elevated number of fibroblasts, a decrease in hyaluronic acid degradation, and an increase in its synthesis by fibroblasts.31 An increase in hyaluronic acid has also been postulated in the initial phases of plaque erosion.32 All of these changes could in turn induce endothelial dysfunction and some degree of systemic inflammation.33,34 These findings could explain the relationship between hypothyroidism and SCAD, with thrombus formation at the media-adventitia interface, a greater tendency for relapse, and lower spontaneous resolution during follow-up. However, further studies are necessary to confirm the importance of the association between SCAD and hypothyroidism and to establish the physiopathological mechanisms involved. Coronary arteries with a”corkscrew“appearance seem to be more frequent in patients with SCAD and have been associated with prognosis in some series. In this series, the SCADs associated with hypothyroidism more frequently had corkscrew coronary arteries but were not associated with worse prognosis.
FMD has been associated with an increased risk of SCAD. In FMD, there is an increase in fibroblasts in the arterial media with elevated collagen synthesis that progressively replaces the muscle cells and weakens the arterial wall.35 In this series, the prevalence of thyroid hormone alterations was not significantly higher in patients with SCAD affected by FMD.
LimitationsAlthough this work involves a considerable number of patients with SCAD (the largest Spanish series to be published), a clear limitation of the study is its small sample size, which particularly affected the comparisons between the different subgroups. In addition, the group without SCAD used as a control was retrospectively obtained from a catheterization database and at a different time point, which could indicate a selection bias. Moreover, SCAD is not an easy entity to diagnose. Intramural hematomas can easily go unnoticed in patients with ACS. Additionally, coronary angiography was not performed with masking of the clinical diagnosis. Finally, FMD was not systematically studied in a small percentage of patients in this series. Taken together, the results of this work should simply be considered hypothesis generators and new studies are required to confirm these findings.
CONCLUSIONSThere is a high prevalence of hypothyroidism in unselected consecutive patients with SCAD. Patients with SCAD and hypothyroidism are more likely to be women and to have corkscrew coronary architecture and more distal dissections that are treated more conservatively. Although preliminary, the data obtained indicate that hypothyroidism could be involved in the pathogenesis of SCAD.
CONFLICTS OF INTERESTNone declared.
- –
SCAD is a rare and often underdiagnosed condition. Many possible triggers or predisposing factors have been described. Its possible association with thyroid function alterations has not been studied. However, hypothyroidism has been associated with an increase in arterial dissections in other “noncoronary” territories, in addition to an increase in events during coronary intervention.
- –
This study reveals an elevated prevalence of hypothyroidism in patients with SCAD. Patients with SCAD and hypothyroidism are more likely to be women, have more distal and diffuse dissections and dissections in corkscrew segments, and be treated more conservatively.