Spontaneous coronary artery dissection (SCAD) is defined as an acute separation of the layers of the coronary artery produced by a cause that is neither traumatic nor iatrogenic. The ensuing accumulation of blood creates a false lumen, which can compress the true lumen, resulting in a decrease in blood flow that leads to myocardial ischemia. The pathophysiology of this condition is uncertain, and 2 possible mechanisms have been proposed. In the first, rupture of the endothelial-intimal layer allows blood from the lumen to enter the medial layer of the artery (“inside out” mechanism). In the second, an initial arterial wall bleed from the vaso vasorum creates an intramural hematoma (“outside in” mechanism). If the pressure within the hematoma increases, secondary endothelial rupture can occur, with communication to the vessel lumen. Therefore, the finding of an entrance portal on imaging study is not specific for the first mechanism.
The exact incidence of SCAD is unknown, as it is usually detected on imaging. In the latest published series and recent consensus documents from international societies,1,2 the incidence is described as 0.07% to 0.2% of all coronary angiograms and in studies performed in patients with an acute coronary syndrome (ACS), it is 1% to 4%. The incidence is known to be higher in women (nearly 90% of cases). SCAD is one of the most common causes of ACS in women younger than 50 years (up to 25%) and it accounts for 34% of cases of acute myocardial infarction in women. Finally, it is the most frequent cause of acute myocardial infarction in peripartum women. SCAD has been related to several conditions, such as fibromuscular dysplasia, pregnancy-related factors, connective tissue diseases, and recently, hypothyroidism.3,4
In a recent article published in Revista Española de Cardiología, García-Guimaraes et al.5 present data from a registry compiled over 4 years in 31 centers. It is one of the largest series of this type and provides a very interesting snapshot of SCAD in our setting. It confirms the demographic data with regard to patient age (53 years) and sex (88% women), as well as the main clinical presentation, an ACS (92%).
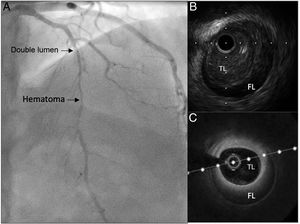
As ACS is the most common presentation form, coronary angiography is usually the first diagnostic imaging method used, and several patterns have been described.6 Definite confirmation would require an intravascular imaging technique, such as intracoronary ultrasound or optical coherence tomography (figure 1). To perform these techniques, an intracoronary guidewire must be passed through the dissection area, which implies a risk of complications that could lead to a loss of coronary flow.7 Therefore, they should be reserved only for cases with inconclusive angiography findings, and should be carried out by operators experienced in coronary interventional procedures and in the interpretation of intracoronary images.
The therapeutic approach is crucial. The course of SCAD is usually toward resolution; hence, the accumulated experience and consensus documents1,2 recommend an initial conservative strategy. Percutaneous coronary interventions (PCIs) should be limited to patients with refractory ischemia and documented coronary occlusion, ventricular arrhythmia, or hemodynamic instability. PCI has a worse outcome in this type of lesion than in atherosclerotic lesions8,9 and there is a potential risk of complications: iatrogenic dissection on the SCAD, guidewire passage to the false lumen, propagation of the intramural hematoma, or untreatable distal dissection. Some authors suggest performing a PCI that differs from conventional procedures: for example, attempting to recover distal flow only by balloon angioplasty, using cutting balloon angioplasty to fenestrate the false lumen and decrease the internal pressure, thereby avoiding propagation of the lesion, or using state-of-the-art drug-eluting stents instead of metal stents. The study by García-Guimaraes et al.5 clearly supports the recommendations, as 78% of patients received conservative management, which was shown to be an independent predictor of a good clinical outcome.
Once an initial conservative strategy has been established, the medical treatment provided is very important. The greatest controversy is related to antithrombotic therapy, which may differ from that recommended for ACS with an atherosclerotic cause, in which dual antiplatelet therapy plus anticoagulant therapy is the standard indicated. This recommendation is based on the fundamental role of the accelerated atherothrombotic cascade and resulting intracoronary thrombus in reducing the distal coronary flow. In contrast, recent studies using optical coherence tomography10 have shown that the presence of thrombi is limited in SCAD, occurring in 36% of cases of fenestrated false lumen and 14% of nonfenestrated SCAD; hence, intensive antithrombotic therapy is not as vital in these patients. Even if the most common pathophysiologic mechanism is intramural hematoma due to vasa vasorum bleeding as many authors believe, intensive treatment may still be detrimental.11 The more potent antiplatelet agents, such as prasugrel, ticagrelor, or glycoprotein IIb/IIIa receptor inhibitors, are clearly discouraged. The present registry shows that although PCI was carried out in 26% of patients (for whom dual antiplatelet therapy would be indicated), up to 59% of patients were prescribed dual antiplatelet therapy at discharge, which would imply a 33% excess of cases with respect to the recommendations.
The role of systemic anticoagulation is even more controversial. The current recommendation is that once SCAD has been diagnosed and there are no other specific indications for anticoagulation (intracoronary thrombus, atrial fibrillation, systemic thromboembolism), this therapy should be immediately discontinued.12
The prognosis of SCAD is quite benign in terms of survival. Ten-year survival is 92.3% in the series with the longest follow-up,13 and 3-year survival is 98.8% in the series with the most patients.14 The prognosis is not so benign with regard to morbidity. Cardiovascular events have been described in 20% to 47.9% of patients depending on the length of follow up, and reinfarction is the most common event. Even more important, the 3-year recurrence rate of SCAD is between 17% and 22%. It is difficult to interpret this datum, as it includes both progressions of the initial dissection and new phenomena in different territories occurring at least 30 days after the first episode. Certain factors predictive of recurrent dissection have been found, such as hypertension14 and coronary artery tortuosity,15 whereas beta-blocker therapy has shown a protective effect.14
As has been mentioned, the most common outcome is resolution of the problem; hence, it is important to be able to verify this after symptom resolution. Unlike the initial phase, in which the severity of the patient's clinical state warrants investigation by invasive coronary angiography, several authors have indicated the usefulness of noninvasive coronary angiography by multislice computed tomography for follow-up.16 Thus, as SCAD more commonly affects young women who can experience menorrhagia while under antiplatelet therapy,2 it has been proposed that detecting “cure” of this condition can safely justify discontinuation of all antithrombotic therapies.
A recent study analyzing ventricular function at long-term using cardiac magnetic resonance has confirmed that SCAD has a relatively benign clinical course.17 The overall ejection fraction decrease is generally mild (57%), and late gadolinium enhancement is absent in 39% of cases.
In summary, SCAD should be suspected in young women with an ACS. Invasive coronary angiography should be used for confirmation in the acute phase, whereas specific intracoronary diagnostic techniques should be performed only in inconclusive cases. The initial therapeutic strategy should be a conservative approach, with PCI being reserved for patients with occlusion of the distal coronary flow. Medical treatment differs from that used for SCAD with an atherosclerotic etiology, as the recommendations discourage intensive antithrombotic therapy. To confirm resolution, multislice computed tomography of the coronary vessels can be the technique of choice. The long-term treatment is uncertain: although SCAD-related mortality is very low, there may be a high recurrence rate.
CONFLICTS OF INTERESTThe authors declare that there are no conflicts of interest related to this Editorial.