Pulmonary embolism (PE) is a common and potentially lethal clinical entity, and one in which cardiologists play an integral role in diagnosis and treatment. Pulmonary embolism is believed to be the third most common cause of cardiovascular disease and death, trailing only myocardial infarction and cerebrovascular disease.1 Epidemiologic modeling suggests an annual incidence of PE exceeding 430 000 in the European Union, with more than a half million venous thromboembolism-related fatalities.2
Despite its broad impact on public health and acute cardiovascular care, the current state of the medical literature on PE is limited compared with either ischemic heart disease or stroke. There are fewer randomized clinical trials and data on prognostics and therapeutics to guide clinicians. Akin to acute coronary syndrome, the urgency and intensity of treatments for PE are gradated based on the degree of arterial occlusion (comparable to nonST-segment myocardial infarction vs ST-segment myocardial infarction), and the severity of hemodynamic and cardiopulmonary sequelae. Risk-stratification guidelines in PE are, however, generally only supported by expert consensus or case series and are not universally consistent. The European Society of Cardiology (ESC) risk stratification,1 as well as that of the American Heart Association 2011 scientific statement,3 delineates patients as high-risk if shock or hypotension is present, and as intermediate risk if the patient remains normotensive but has evidence of either myocardial necrosis or right ventricle (RV) strain. Such evidence may include elevated troponin, elevated natriuretic peptides, electrocardiographic changes, or imaging evidence (echocardiographic or computed tomographic) of RV dilatation, RV dysfunction, elevated pulmonary pressures, and/or interventricular septal compression.4 The ESC updated its schema in 20141 after publication of the vitally important PEITHO trial,5 and now separates intermediate-risk patients into ⿿intermediate-high⿿ risk if there is both biomarker evidence of myocardial necrosis and imaging evidence of RV strain; ⿿intermediate-low⿿ risk patients have only 1 of these elements present. The current guidance from the American College of Chest Physicians mainly differentiates ⿿PE with hypotension⿿ from ⿿PE without hypotension.⿿6
While interventions for acute coronary syndrome have matured to focus on angioplasty and stenting, multiple novel potential reperfusion therapies are being developed and tested for PE.7 Treatment strategies include systemic fibrinolysis, catheter-directed fibrinolysis (CDF), mechanical aspiration and maceration of thrombi, combination ⿿pharmacomechanical⿿ approaches, surgical pulmonary embolectomy, and adjunct use of mechanical circulatory support (Figure 1). Limited data are available in terms of PE trials with large patient populations and extended follow-up. American College of Chest Physicians and American Heart Association guidelines, even for the highest-risk patients, provide class II recommendations for use of various interventional therapies. The ESC guidelines now give class I recommendations for consideration of systemic thrombolysis in high-risk patients, or if contraindicated, surgical pulmonary embolectomy. However, most of the guidelines for treatment of intermediate-risk patients (aside from anticoagulation) are class II and supported by level B or C evidence.1 In practice, therapeutic decision-making in PE is not generally standardized, but rather rendered on an individual basis depending on institutional practice and local expertise.
Reperfusion therapy in pulmonary embolism. Reperfusion therapies for pulmonary embolism include systemic thrombolysis, percutaneous methods (which include synergy with infusions of thrombolytics, ultrasound-assisted thrombolysis, and other clot disruption methods), surgical pulmonary embolectomy, and mechanical approaches to supporting the right ventricle such as VA ECMO, or possibly novel percutaneous assist devices (eg, Impella RP). CDF, catheter-directed fibrinolysis; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Modern management of certain conditions like acute coronary syndrome often funnels patients directly to cardiologists or specific teams. In contrast, PE patients interface with the acute health-care system in heterogeneous ways. Many medical specialties encounter PE, including ambulatory physicians, emergency medicine, internists and hospitalists, oncologists, obstetricians, and surgeons. Historically, in transactional models of care, this referring physician may choose to consult with a single cardiologist, surgeon, or interventional radiologist for treating PE. The referring physician likely would have to serially consult several specialties in order to access available therapeutic options, but there would generally be no overarching opinion or specific guidance on optimal strategy. Confounding matters, some PE patients require urgent evaluation and decision-making without time for serial consultations.
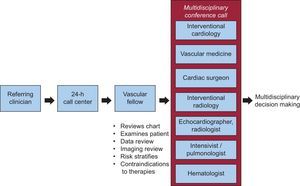
To both expedite such complicated consults and leverage the experience of all of the various specialties involved in the care of PE, several medical centers have instituted multidisciplinary pulmonary embolism response teams (PERT) (Figure 2).8 The first PERT was founded in 2012 at Massachusetts General Hospital (MGH) in Boston.8 Weill Cornell established their Pulmonary Embolism Advanced Care (PEAC) team in 2013. The fundamental concept of PERT involves mobilizing multiple specialists, for expedited assessment of intermediate and high-risk PE patients, toward effectuating a coordinated treatment plan. Such teams necessarily require the expertise of multiple practitioners, including experts in vascular medicine and venous thromboembolism evaluation (hematology), diagnostic imaging (echocardiography, radiology), percutaneous and surgical interventions (interventional cardiology, interventional radiology, and cardiothoracic surgery), and the care of the acutely ill patient (emergency medicine, intensive care, pulmonology).
Pulmonary embolism response team (PERT) activation. A PERT is activated by a call center, which activates a ⿿front-line⿿ clinician (usually a fellow in vascular medicine or critical care medicine) who performs an expedited review of data, imaging, and patient examination, in order to risk-stratify pulmonary embolism patients, assess their trajectory, gauge the risks of various interventions, and inquire about the patients⿿ goals and preferences. Then a virtual conference is held to discuss the case with all of the specialists of the PERT; the goal of the conference is to confirm risk stratification and plan the best possible treatment strategy.
The philosophical and operational background of PERT derives from 2 concepts in modern systems-based cardiovascular care: the heart team and the rapid response team.9 The heart team concept is becoming prominent in modern cardiology10 as a guidelines-endorsed component of medical decision-making in complex percutaneous coronary intervention,11 transcatheter aortic valve replacement,12 and stroke.13 The heart team facilitates cognitive interchange beyond the confines of each component discipline and is designed to generate a consensus opinion of the members who together work to assess patient-specific risks and benefits of possible alternative treatment strategies in the absence of clinical trial data that affords a clear answer.9 Therefore, decision-making based on a heart team model is considered a quality marker in cardiology.14 A multidisciplinary heuristic may be more able to balance risks of intervening vs not-intervening, especially when there are multiple novel devices and approaches that have not yet been rigorously tested in randomized trials. Moreover, certain endovascular and surgical treatments for PE also require multiple types of physician expertise in conjunction with the interventionalist (eg, transesosphageal echocardiography, anesthesiology).
Rapid response teams institute a protocolized framework to react to predictable patient-decompensation scenarios and have been shown to reduce in-hospital mortality after eg, cardiopulmonary arrest. Each system requires an activation limb with criteria to activate and notify the team, and an effector limb with the predefined action of medical specialists.9 Rapid response literature also endorses creating a robust administrative infrastructure and continuous quality improvement initiatives as key components of sustaining a successful program.15
PULMONARY EMBOLISM RESPONSE TEAM: SETUP AND INITIATIONPulmonary embolism response teams at MGH and Weill Cornell are composed of specialists each with self-identified interest in treating PE patients: clinician motivation and dedication are key facets to the success of any PERT. The ideal clinician-participant will be one who will feel comfortable joining in a shared-decision making model with other clinicians.
While some heart teams will have scheduled, elective meetings, a PERT by definition requires impromptu meetings to respond to ill patients, just like ST-segment myocardial infarction or stroke teams. As PERT is modeled on rapid response philosophy, pre-existing rapid response teams at the hospitals provide a familiar analogue to the PERT concept for clinicians both requesting and providing the PERT consultant service. Nevertheless, before the initial launch, significant efforts at intramural education should include presentations at departmental conferences and grand rounds, quality improvement efforts, and for example color posters documenting the PERT algorithm provided to inpatient floors and the emergency ward. Targeting practice groups such as emergency and intensive care is essential as a relatively high proportion of PE patients arise from these locations; engaging these physicians is essential to the success of a PERT, and having an effective partnership and agreement on institutional protocol ensures that acutely ill patients will quickly receive the consultation and therapies indicated.
The major ⿿inputs⿿ to a PERT team reflect the availability of key clinical services, including 24-h emergency services, radiology, echocardiography, catheterization laboratories, and operating room access.8 Not all hospitals will have all portions of this infrastructure, and so in creating a PERT each hospital will have to tailor its ability to respond to existing resources. The Massachusetts General Hospital PERT and Weill-Cornell PEAC each also heavily rely on physicians-in-training to serve as the ⿿front-line⿿ clinician, and at nonteaching hospitals the initial triage and consult role may have to be undertaken by staff physicians. Support of hospital leadership and administration is another key factor in launch.
PULMONARY EMBOLISM RESPONSE TEAM: OPERATIONSPulmonary embolism response teams can be activated by any clinician via a call to a 24-h call center. A PERT physician, typically a fellow in vascular medicine or critical care, expeditiously gathers clinical information by discussion with the primary team, electronic health record review, and evaluation of the patient; the goal of this evaluation is to perform a PE risk-stratification, with close attention paid to hemodynamic status, cardiopulmonary stability, right heart strain, and contraindications to interventions.4,9 For cases with a specific therapeutic or management question, the fellow notifies the remainder of the multidisciplinary PERT team, who convene an online meeting using commercial software (eg, GoToMeeting16). During this conference, the patient's case history, imaging findings, and assessments are reviewed and a consensus treatment plan is formulated in real-time, taking account of the individual patient's presentation, trajectory, comorbidities, and preferences and treatment goals. Typically about 8 to 10 physicians join in a virtual meeting lasting 15min to 20min. The referring clinician and patient's current attending are also invited to join. Afterward, the PERT fellow summarizes and communicates therapeutic recommendations (with a goal of completing this process within 90minutes from receipt of the consult), and if necessary, mobilizes special resources for the treatment plan (eg, catheterization suite).
The PERT was also created with core aims of data collection, research to improve the care of PE patients, and process and quality improvement from the beginning. Both the MGH PERT and Weill Cornell PEAC have established web-based prospective data collection tools, compliant with existing health privacy laws, to track patient data on all PERT cases. Clinical research staff and PERT fellows import data in real-time during a patient's hospital course, and data is collected at 7 days, 30 days, and 365 days after consultation.
Following the consultation, the PERT team will follow-up the patient during the hospitalization. Massachusetts General Hospital PERT and Weill Cornell (⿿Thrombosis Clinic⿿) each conduct a monthly clinic for expedited and focused PE follow-up (usually within a few weeks of the index event). These clinics are staffed jointly by vascular medicine, pulmonology, and hematology, as well as by interventional cardiology and/or cardiac surgery (if interventions were performed). An integrated follow-up clinic facilitates understanding of outcomes in PE and postintervention and supports research aims.
PERFORMANCE: MASSACHUSETTS GENERAL HOSPITAL PULMONARY EMBOLISM RESPONSE TEAMThe MGH PERT has in 3.5 years of operation received consultation requests on over 600 patients. Consults originate primarily from emergency services (55%) with about half of the remainder coming each from intensive care units and hospital floors. Pulmonary embolism response teams may also be empirically consulted for a deteriorating patient without a clearly-defined etiology because clinicians may suspect PE on the differential diagnosis; in the MGH experience, only about 80% of patients for whom PERT consultation was requested were proven to have PE.17 In the Weill Cornell experience, evaluations by the PEAC fellow in a deteriorating patient have also led to diagnoses of tamponade requiring urgent intervention, or decompensated heart failure requiring mechanical support and transplant evaluation. At MGH, in about one third of confirmed-PE cases, the consulting fellow addresses the treatment question without need to involve the entire multidisciplinary PERT; however, the remaining 305 cases were presented via online conference to the full MGH PERT in real-time. Recommended treatment after PERT consultation is predominantly anticoagulation alone, with two thirds of patients-given this recommendation. One sixth of patients are recommended to have inferior vena cava filters placed (generally due to contraindications to anticoagulation), whereas about 12% receive thrombolysis due to more severe hemodynamic and cardiopulmonary compromise, of which 3% were systemic thrombolysis and 9% were CDF. These data are analogous to existing PE registry data describing use of thrombolysis (eg, 13% thrombolysis in the International Cooperative Pulmonary Embolism Registry), though there is a tendency toward more catheter-based treatment in the MGH series. Major bleeding complications in the MGH cohort were the same in patients receiving CDF vs those receiving anticoagulation alone (4% each).17 However, robust efficacy and cost-benefit data of this approach is lacking. Overall survival to discharge after an MGH PERT consult is 87%.
PULMONARY EMBOLISM RESPONSE TEAM: CHALLENGESOutcome, cost-effectiveness, and quality data on PERT teams and their results do not yet exist; understandably, PERTs are not yet discussed in any professional society guideline. Data generated from PERT for the short-term will be in the form of registry and cohort data, although more rigorous clinical experience will ultimately be required to prove the benefit of the concept.
Pulmonary embolism response team models to date have operated fluidly based on the interest and goodwill of participant physicians. Involved physicians are effectively subscribing for additional 24-h call as consultations may arise at any time, on top to their routine clinical practice. In the traditional model of serial consultation that PERT has sought to improve upon and replace, all specialty physicians are reimbursed for their role and evaluation. While compensation models are in evolution for heart teams, in the current iteration of MGH PERT, only the supervising PERT staff physician receives compensation for evaluation and management services. Thus one of the indirect costs of PERT includes, from the point-of-view of a fee-for-service model, uncompensated time of the PERT team physicians. However, PERT may fit well into future capitated payment models, especially if such a team improves outcomes for the higher-risk PE patients or reduces length of intensive care unit and/or hospital stay.
Finally, over-reliance on the novel technologies for PE treatment was a possible side effect of structuring a PERT with physician experts in interventions: this was not observed in the MGH experience, but a true assessment of the ⿿appropriate use⿿ of PE interventional therapy will have to wait for robust outcome and cost data.
CONCLUSIONBecause clinical data and guidelines do not cover all scenarios in treating intermediate and high risk PE, the PERT concept is designed to apply multispecialty cognitive and procedural expertise to these patients. Pulmonary embolism response team combines the philosophies of the heart team and rapid response team to generate a prompt, patient-specific plan for candidate patients without needing to consult multiple individual physicians. Before launching a PERT, institutions should evaluate participation of the necessary component physicians, manpower which often involves physicians-in-training in fellowship programs, administrative support, and a technological mechanism to facilitate real-time multiphysician communication and consultation. Operational challenges to begin and sustain PERT include publicizing the initiative both intramurally and in local networks of partner and referring hospitals, routine meetings of involved physicians to promote continuous education in this evolving space, ongoing quality analyses, and longitudinal and outpatient follow-up. Efficacy data and cost analyses will be required to validate the PERT concept.
CONFLICTS OF INTERESTNone declared.