The occurrence of stent thrombosis (ST) during follow-up remains as the most feared complication after percutaneous coronary intervention (PCI). ST is associated with dreadful outcomes, with previous studies reporting mortality rates of up to 30%.1 While the advent of drug-eluting stents (DES) significantly outperformed bare metal stents in terms of restenosis, initial reports showed that early-generation DES (early-DES) were associated with an increased risk of ST.2 Subsequently, the introduction of new-generation DES (new-DES) with thinner struts and biocompatible durable or biodegradable polymers (and polymer-free stents) reduced ST rates, particularly those of very late ST (VLST).3 However, data comparing ST rates between early and new-DES at very long-term follow-up are scarce.
In a recent article published in Revista Española de Cardiología, Coughlan et al.4 contributed with important data on this field. The DECADE cooperation is a pooled analysis of individual patient data from 5 DES trials with 10-year follow-up.5–9 Patients were divided in 2 groups (early- and new-DES) and the primary endpoint was definite ST through 10 years after PCI. This multicenter collaboration included 9700 patients, 6866 in the new-DES group and 2834 in the early-DES group. Of note, patients in the new-DES group were older and more frequently had diabetes and multivessel disease. In addition, there was a higher proportion of complex and bifurcation lesions in the new-DES group. All patients were prescribed a dual antiplatelet regimen of indefinite aspirin and clopidogrel for 6 to 12 months after PCI.
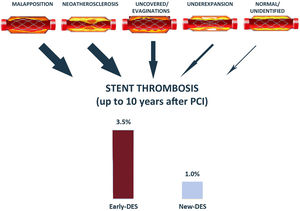
The main results of the study can be summarized as follows: a) in this pooled analysis of 5 multicenter trials, the cumulative incidence of definite ST at 10 years of follow-up using new-DES was 1%; b) definite ST occurred less frequently in new-DES than in early-DES (1% vs 3.5%; adjusted hazard ratio [HR],=0.32; 95% confidence interval [95%CI], 0.23-0.45), especially regarding VLST (0.3% vs 1.5%; adjusted HR,=0.16; 95%CI, 0.09-0.28) and very very late ST (> 5 years after PCI) (0.2% vs 0.9%; adjusted HR,=0.25; 95%CI, 0.23-0.45). This is the first pooled study comparing ST between early- and new-DES at 10 years of follow-up and represents the larger cohort focusing on very late outcomes using new-DES.
The authors should be congratulated for this cooperative effort with significant clinical implications. In addition, some aspects of the study by Coughlan et al. work merit further discussion. First, the present study4 is largely an indirect comparison since only 2 out of the 5 trials included directly compared early-DES vs new-DES and, most early-DES patients came from older studies. Thus, outcomes could also have been improved by implantation techniques and better medical treatment (ie, intensive therapy with rosuvastatin and eicosapentaenoic acid have been shown to prevent in-stent neoatherosclerosis,10 which accounts for one third of VLST, and a combination of rosuvastatin and alirocumab encourages plaque stabilization and reduces myocardial infarction11). Moreover, an optical coherence tomography study of patients with VLST (median time from implantation 4.8 years) showed minimal differences between early- and new-DES.12 Second, the main endpoint did not include data on probable or possible ST and was limited to definite ST, which may have partially underestimated the incidence of ST. Importantly, the SIRTAX trial,8 which included one third of patients in the early-DES group had a different definition of ST. Nevertheless, the current analysis has strong clinical value and adds an important piece of information in this context.
ST was a major concern with the introduction of first-generation DES, and the initial reports showed no clear evidence of risk attenuation of ST beyond the first year after PCI. Although the etiology of VLST is multifactorial, the inflammatory response to the durable polymer used in early-DES did indeed seem to play a significant role. In the present work, Coughlan et al. demonstrated that the enhancements included in new-DES seemed to reduce definite ST at long-term follow-up compared with early-DES. Of note, the cumulative incidence of definite ST was low (1% at 10 years), and a sustained attenuation of its occurrence was observed beyond 1 year following PCI.2 The latter adds reassuring data regarding late clinical outcomes after PCI using current-generation DES.
In the present analysis, no differences in ST were observed between early- and new-DES during the first year after PCI, both subacute (< 1 month) and late (from 1-12 months) ST.4 Although this could be explained by β-error, it may suggest that, contrary to VLST, its occurrence within the first few months after PCI (specially within the first weeks) could be more strongly influenced by intraprocedural and lesion factors, baseline patient risk, and antithrombotic treatment. By contrast, the main causes related to VLST are, by order: malapposition (∼35%), neoatherosclerosis (∼30%), uncoverage (10-15%), underexpansion (5-10%), other (edge disease, restenosis, etc) (∼5%)12 (figure 1). Thus, although lack of polymer toxicity and thin struts could explain a significant reduction in VLST, it should be acknowledged that modern implantation techniques and intensive therapy could also have played a role in this reduction in events.
On the other hand, the duration of dual antiplatelet therapy along with the use of more potent P2Y12 inhibitors is key regarding clinical outcomes during the first few months after PCI. In the present work, neither prasugrel nor ticagrelor were used, which could have decreased the rate of ST during the first year following PCI. Moreover, the very low rate of ST in the long-term with current implantation techniques and treatment are aligned with the current trend of decreasing dual antiplatelet therapy duration after PCI.13 Such a low event rate up to 10-years follow-up would not justify the risk of bleeding associated with prolonged duration of dual antiplatelet therapy.
Importantly, DES technology continues to improve. Modern ultra-thin (< 60μm) DES have outperformed the new-DES included in the present analysis,14 and second-generation DES with enhanced drug-release technology have demonstrated better clinical outcomes than other contemporary stent platforms in high-risk subsets such as diabetic patients.15,16
In conclusion, the work by Coughlan et al.4 adds new information on the safety profile of new-DES at long-term, with low incidence of definite ST through 10 years of follow-up. While the global management of patients with coronary artery disease should continue to improve, this multicenter cooperation adds reassuring data in the challenging setting of late stent-related outcomes following PCI.
FUNDINGNone
CONFLICTS OF INTERESTR. Romaguera has received speaker honoraria from Medtronic, Biosensors, Alvimedica and Biotronik, and proctoring fees from Boston Scientific. The other authors declare no conflicts of interest.